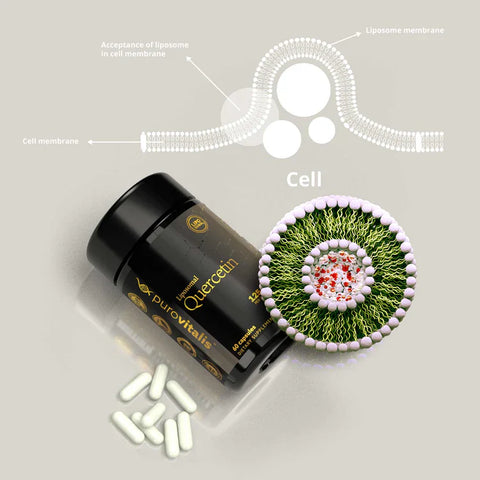
Learn essential supplementation skills with our guide on the top three natural senolytics that can rejuvenate cells and enhance health. As we age, cellular senescence—where cells stop dividing and accumulate in our bodies—can lead to various age-related diseases. However, certain natural compounds have been identified as powerful senolytics capable of selectively clearing these aged cells. This article looks deeper into the science behind these remarkable substances and how to incorporate them into daily routines for age-defying health benefits.
Introduction
Aging is a complex biological process characterized by a gradual decline in physiological function and increased susceptibility to diseases. While aging has long been viewed as an inevitable aspect of life, recent advances in biogerontology have uncovered cellular and molecular mechanisms underlying it. Cellular senescence has emerged as a prominent contributor to age-related pathologies among these mechanisms. It is an irreversible growth arrest triggered by various stressors.(1)
Because the number of senescent cells increases with aging, it has been presupposed that senescence contributes to aging. Senescence is needed to prevent the distribution and proliferation of damaged cells, triggering an immune system response. This cellular checkpoint requires an efficient cell substitution system that involves both clearances of senescent cells and mobilization of progenitor cells to restore optimal cell numbers.(2)
Nuclear DNA damage is often reported as a commonly underlying cause of senescence, mainly in the form of DNA double-strand breaks (DSBs) that activate the DNA damage response (DDR) pathway. Prolonged DDR activation activates senescence. One or a few DDR signaling telomeres (chromosome ends) are sufficient to trigger replicative cell senescence. Oncogene activation is also a strong senescence trigger.(3)
Senescent cells express substantial alterations in their secretome, which is particularly enriched in proinflammatory cytokines and matrix metalloproteinases. It is hence referred to as the senescence-associated secretory phenotype (SASP). Senescent cells exhibit distinct phenotypic changes, such as flattened morphology, altered gene expression and the secretion of proinflammatory molecules. While senescence initially serves as a tumor-suppressive mechanism by halting the proliferation of damaged cells, the accumulation of senescent cells over time contributes to tissue dysfunction and promotes aging-related diseases. (4)
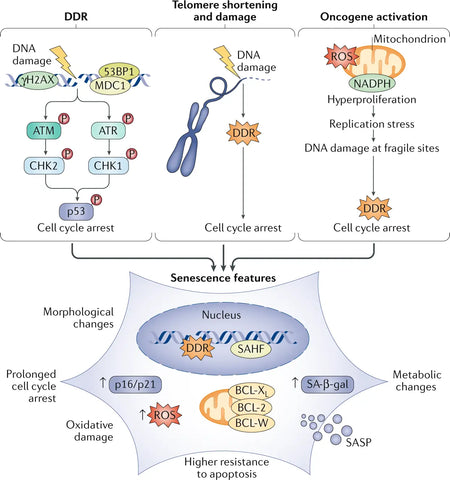
Image: Senescence drivers and phenotypes.
Source: Di Micco, R., Krizhanovsky, V., Baker, D., & d’Adda di Fagagna, F. (2021). Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nature Reviews Molecular Cell Biology 22 (2): 75–95.
Given the detrimental effects of senescent cells on tissue homeostasis and healthspan, targeting these cells has emerged as a promising therapeutic strategy. Senolytics are compounds designed to induce apoptosis in senescent cells while sparing healthy cells selectively. They hold the potential to alleviate inflammation, enhance tissue regeneration and delay the onset of age-related pathologies.(5)
Senolytic Compounds
Numerous natural and synthetic compounds have been identified as potential senolytics, with quercetin (a natural compound) and dasatinib (a synthetic drug) representing early candidates in this class. In addition to quercetin and dasatinib, several other compounds, including fisetin, navitoclax and ABT-263, have shown promising senolytic properties in preclinical models. This article covers the top 3 potential and promising natural senolytics available today.
Fisetin
Fisetin is a bioactive flavonol (a polyphenol) studied considerably for its potential to promote health and longevity, mainly by mitigating cellular senescence. It is found primarily in strawberries, apples, persimmon, onions, grapes and in small amounts in cucumber (see image). Fisetin has a range of biological activities attributable to its unique molecular structure (a planar structure and several carbon rings).(6-7)
Initial exploration into Fisetin's properties reveals its potent antioxidant capacity, which stems from its capability to scavenge reactive oxygen species (ROS). Thus, it plays a crucial role in cellular defense against oxidative stress.(8)
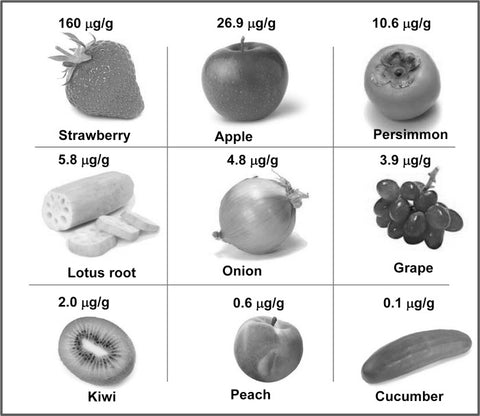
Image: Dietary sources of fisetin.
Source: Khan, N. & Syed, D. & Ahmad, N. & Mukhtar, H. (2013). Fisetin: a dietary antioxidant for health promotion. Antioxidants & Redox Signaling 19 (2): 151–162.
The senolytic activity of Fisetin constitutes a primary focus in longevity research. Cellular senescence is a state where cells cease proliferating and accumulate over time – it is involved in various age-related diseases (see before in more detail). Fisetin has been found to induce apoptosis in these senescent cells selectively. This selective clearance is hypothesized to alleviate senescence-associated phenotypes, thereby contributing to the delay or prevention of age-related pathologies. Compared to another possible senolytic compound, Fisetin is about two times more potent than quercetin (see later in the article).(9-10)
The prooxidant activity of flavonoids (such as fisetin) is an important consideration when screening for senolytics. Senescent cells accumulate high levels of copper and iron. The selective mechanism of quercetin or fisetin is associated explicitly with copper/iron-promoted oxidative damage in senescent cells thus killing apoptosis-resistant cells.(11)
Fisetin also affects crucial cellular signaling pathways that are integral to the aging process. It modulates the function of sirtuins (SIRT1 particularly), mTOR (inhibition) and JAK-STAT/NF-κB, which are crucial in regulating cellular survival, apoptosis and autophagy. By modulating these pathways, Fisetin can theoretically improve cellular function, reduce inflammation, and maintain tissue homeostasis.(12-14)
Fisetin has also been shown to have neuroprotective properties. It mitigates neuronal damage and enhances cognitive functions, primarily through its antioxidative action and modulation of neuronal signaling pathways.(15-16)
Despite these promising preclinical findings, it is essential to acknowledge that most research on Fisetin has been limited to in vitro and animal models. Translating these results to human clinical applications necessitates rigorous clinical trials to ascertain Fisetin's efficacy, safety and optimal dosing. Luckily, some clinical studies are already on the way, and we should have results in the next few years.(17-18)
Quercetin
Quercetin is an important antioxidant that is categorized as a flavonol. It naturally occurs in many vegetables, fruits, berries, leaves and grains. Quercetin is primarily found in capers, radishes, dill, coriander, cabbage, red onion, broccoli and berries such as cranberries and lingonberries. Quercetin is one of the most significant and common flavonols occurring in nature.
Research shows that quercetin acts as an anti-viral, anti-microbial, and anti-inflammatory agent.(19) Moreover, studies demonstrated the antihistamine effects of quercetin, meaning that it may help treat allergies.(20-21) The most significant impact of quercetin on the human body is its ability to reduce silent inflammation.(22)
The potential of quercetin to promote longevity can be attributed to its antioxidant properties. As an antioxidant, quercetin scavenges free radicals, reducing oxidative stress in the body. Research has indicated that quercetin can activate sirtuins (SIRT-1 particularly), which regulate cellular processes like DNA repair, gene expression and metabolism. The activation of sirtuins is linked to increased lifespan in various organisms.(23-24)
Quercetin has also been shown to activate the Nrf2 pathway, and in cell nuclei, it activates the transcription of various antioxidant response element (ARE)-driven genes. These genes upregulate the expression of cytoprotective enzymes, such as glutathione S-transferase, NAD(P)H quinone dehydrogenase 1, and heme oxygenase-1.(25)
Usually, quercetin supplements are taken at a dose of 500 mg twice a day. However, the optimal dosage of quercetin has yet to be determined. The recommended dietary allowance for quercetin is typically 5 to 40 mg daily, but it can also be consumed in significantly more significant amounts (> 500 mg).
Regarding quercetin, our recommendation is the liposomal form of it by Purovitalis.
Apigenin
Apigenin (4′,5,7,-trihydroxyflavone) is a naturally occurring flavonoid compound in various plants, including parsley, celery and citrus fruits. It is particularly abundant in the flowers of the chamomile plant. Apigenin has the potential to aid in preventing chronic diseases like diabetes, Alzheimer's, depression, insomnia and cancer, with benefits observed in vivo research (animal and human studies).(26)

One of the most promising aspects of apigenin is its ability to promote cellular resilience. Apigenin has been shown to activate the Nrf2 pathway, which is crucial in the body's defense against oxidative stress and inflammation. By upregulating the expression of antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase, apigenin helps protect cells from damage caused by reactive oxygen species (ROS).(27)
In addition to its antioxidant properties, apigenin has demonstrated anti-inflammatory effects. It has been found to inhibit the production of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, by modulating the NF-κB signaling pathway.(28) Apigenin has also been investigated for its potential anti-cancer properties. Studies have shown that apigenin can induce apoptosis (programmed cell death) in various cancer cell lines, including breast, prostate, and colon cancer cells. Apigenin also promotes autophagy, thereby aiding in removing dysfunctional cellular elements.(29-30)
Apigenin has also been studied for its ability to modulate pathways associated with aging. One such pathway is the insulin/IGF-1 signaling (IIS) pathway, known to play a role in lifespan regulation across various species. Apigenin has been shown to inhibit the IIS pathway, potentially mimicking the effects of caloric restriction, a well-established intervention for promoting longevity.(31-32) Furthermore, apigenin inhibits the NAD+ase CD38, which is associated with metabolic syndrome, increasing intracellular NAD+ levels and decreasing global protein acetylation.(33) Regarding senescence, in particular, apigenin helps to inhibit remaining senescent cells from making SASP (senescence associated secretory phenotype; see before) to support cellular and tissue health.(34)
Regarding dosing, there is no universally recommended dose for apigenin supplementation. However, studies have used doses ranging from 25 to 100 mg/kg body weight in animal models.(35) Supplements typically range from 50 to 500 mg per day. However, the most effective and safe dosage has yet to be conclusively established. Apigenin is generally considered safe, but high doses may interact with certain medications.
Conclusion
In concluding the analysis of the potential of natural senolytics, it is evident that compounds such as fisetin, quercetin and apigenin exhibit significant senolytic capabilities that may profoundly impact the aging process. These substances specifically target and eliminate senescent cells, thus addressing a fundamental aging mechanism and related pathologies. Integrating these bioactive compounds into one’s diet—derived from sources like strawberries, onions, and chamomile—represents a strategic approach to enhancing cellular function and mitigating the accumulation of cellular damage over time.
However, while the preclinical data are promising, translating these findings into practical clinical strategies requires further empirical research to validate efficacy and safety. As such, the continued exploration of these compounds within rigorous clinical trials remains crucial.
Scientific references:
- Sikora, E., Arendt, T., Bennett, M., & Narita, M. (2011). Impact of cellular senescence signature on ageing research. Ageing research reviews, 10(1), 146-152.
- He, S., & Sharpless, N. E. (2017). Senescence in health and disease. Cell, 169(6), 1000-1011.
- Di Micco, R., Krizhanovsky, V., Baker, D., & d’Adda di Fagagna, F. (2021). Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nature Reviews Molecular Cell Biology 22 (2): 75–95.
- Kuilman, T. & Michaloglou, C. & Mooi, W. & Peeper, D. (2010). The essence of senescence. Genes & Development 24 (22): 2463–2479.
- Kirkland, J. L., & Tchkonia, T. (2020). Senolytic drugs: from discovery to translation. Journal of internal medicine, 288(5), 518-536.
- Sengupta, B. & Banerjee, A. & Sengupta, P. (2005). Interactions of the plant flavonoid fisetin with macromolecular targets: insights from fluorescence spectroscopic studies. Journal of Photochemistry and Photobiology B: Biology 80 (2): 79–86.
- Bag, S. & Ghosal, S. & Karmakar, S. & Pramanik, G. & Bhowmik, S. (2023). Uncovering the Contrasting Binding Behavior of Plant Flavonoids Fisetin and Morin Having Subsidiary Hydroxyl Groups (− OH) with HRAS1 and HRAS2 i-Motif DNA Structures: Decoding the Structural Alterations and Positional Influences. ACS Omega 8 (33): 30315–30329.
- Khan, N. & Syed, D. & Ahmad, N. & Mukhtar, H. (2013). Fisetin: a dietary antioxidant for health promotion. Antioxidants & Redox Signaling 19 (2): 151–162.
- Yousefzadeh, M. et al. (2018). Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36: 18-28.
- Wyld, L. et al. (2020). Senescence and cancer: a review of clinical implications of senescence and senotherapies. Cancers 12 (8): 2134.
- Wang, Y. & He, Y. & Rayman, M. & Zhang, J. (2021). Prospective selective mechanism of emerging senolytic agents derived from flavonoids. Journal of Agricultural and Food Chemistry 69 (42): 12418–12423.
- Wiciński, M. et al. (2023). Natural Phytochemicals as SIRT Activators—Focus on Potential Biochemical Mechanisms. Nutrients 15 (16): 3578.
- Afroze, N. et al. (2022). Fisetin deters cell proliferation, induces apoptosis, alleviates oxidative stress and inflammation in human cancer cells, HeLa. International Journal of Molecular Sciences 23 (3): 1707.
- Roy, T. et al. (2023). Dual targeting of mTOR/IL-17A and autophagy by fisetin alleviates psoriasis-like skin inflammation. Frontiers in Immunology 13: 1075804.
- Samanta, S. et al. (2022). The neuroprotective effects of fisetin, a natural flavonoid in neurodegenerative diseases: Focus on the role of oxidative stress. Frontiers in Pharmacology 13: 1015835.
- Singh, S. & Singh, A. & Garg, G. & Rizvi, S. I. (2018). Fisetin as a caloric restriction mimetic protects rat brain against aging induced oxidative stress, apoptosis and neurodegeneration. Life Sciences 193: 171–179.
- Verdoorn, B. et al. (2021). Fisetin for COVID‐19 in skilled nursing facilities: Senolytic trials in the COVID era. Journal of the American Geriatrics Society 69 (11): 3023–3033.
- Kirkland, J. (2024). Alleviation by Fisetin of Frailty, Inflammation, and Related Measures in Older Women (AFFIRM). ClinicalTrials.gov ID: NCT03430037.
- Chirumbolo, S. (2010). The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflammation & Allergy-Drug Targets 9 (4): 263–285.
- Chirumbolo, S. (2011). Quercetin as a potential anti-allergic drug: which perspectives? Iran Journal of Allergy Asthma and Immunology 10 (2): 139–140.
- Sagit, M. et al. (2017). Effectiveness of quercetin in an experimental rat model of allergic rhinitis. European Archives of Oto-Rhino-Laryngology 274 (8): 3087–3095.
- Li, Y. et al. (2016). Quercetin, Inflammation and Immunity. Nutrients 8 (3): 167.
- Costa, L. & Garrick, J. & Roquè, P. & Pellacani, C. (2016). Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxidative Medicine and Cellular Longevity 2016: 2986796.
- Cui, Z. et al. (2022). Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Frontiers in Immunology 13: 943321.
- Suraweera, T. & Rupasinghe, H. & Dellaire, G. & Xu, Z. (2020). Regulation of Nrf2/ARE Pathway by Dietary Flavonoids: A Friend or Foe for Cancer Management? Antioxidants 9: 973.
- Salehi, B. et al. (2019). The therapeutic potential of apigenin. International Journal of Molecular Sciences 20 (6): 1305.
- Paredes‐Gonzalez, X. et al. (2015). Induction of NRF2‐mediated gene expression by dietary phytochemical flavones apigenin and luteolin. Biopharmaceutics & Drug Disposition 36 (7): 440–451.
- Ginwala, R. & Bhavsar, R. & Chigbu, D. & Jain, P. & Khan, Z. (2019). Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 8 (2): 35.
- Shukla, S. & Gupta, S. (2010). Apigenin: a promising molecule for cancer prevention. Pharmaceutical Research 27: 962–978.
- Sung, B. & Chung, H. & Kim, N. (2016). Role of apigenin in cancer prevention via the induction of apoptosis and autophagy. Journal of Cancer Prevention 21 (4): 216–226.
- Pan, H. & Finkel, T. (2017). Key proteins and pathways that regulate lifespan. Journal of Biological Chemistry 292 (16): 6452–6460.
- Shukla, S. & Gupta, S. (2009). Apigenin suppresses insulin‐like growth factor I receptor signaling in human prostate cancer: An in vitro and in vivo study. Molecular Carcinogenesis 48 (3): 243–252.
- Escande, C. et al. (2013). Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes 62 (4): 1084–1093.
- Lim, H. & Park, H. & Kim, H. (2015). Effects of flavonoids on senescence-associated secretory phenotype formation from bleomycin-induced senescence in BJ fibroblasts. Biochemical Pharmacology 96 (4): 337–348.
- Salehi, B. et al. (2019). The therapeutic potential of apigenin. International Journal of Molecular Sciences 20 (6): 1305.