The main physiological mechanism affecting the stability and general state of alertness during the working day is the regulation of blood sugar. Maintaining a stable level of blood sugar by refraining from overeating, continuous snacking and frequent meals is key to productivity and clarity of mind.
Constant spikes and crashes in blood sugar are significant factors in mood swings. Hypoglycemia (low blood sugar) in particular can cause anxiety, irritability, and edginess, usually preceded by a significant drop in cognitive performance. Conversely, for diabetics, a high level of blood sugar is associated with impaired cognitive performance and negative moods. Blood sugar can reach a low level even in healthy individuals, for example, due to prolonged exercise or fasting.
The activation of hunger signals is not necessarily preceded by a hypoglycemic (physiologically low) blood sugar level – usually, the sensation of hunger and the urge to eat is triggered by a rapid decrease in blood sugar. Thus, maintaining a constant level of blood sugar reduces hunger pangs and helps achieve a stable state of alertness throughout the working day.
HORMONES INVOLVED IN BLOOD SUGAR REGULATION
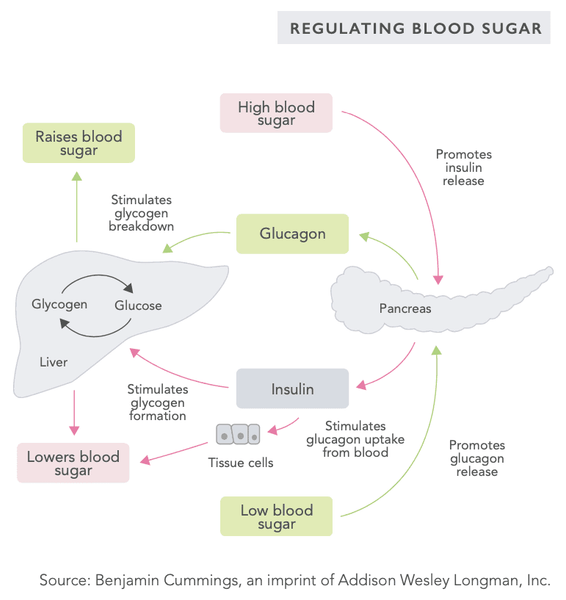
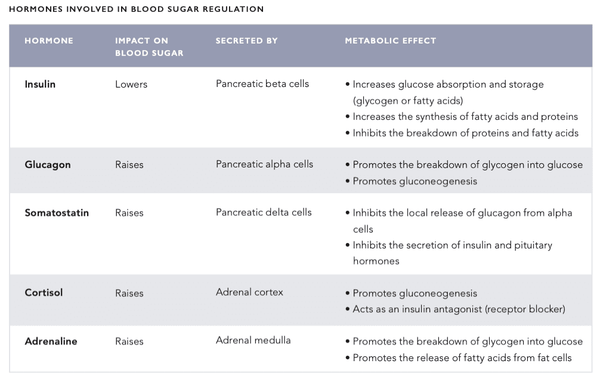
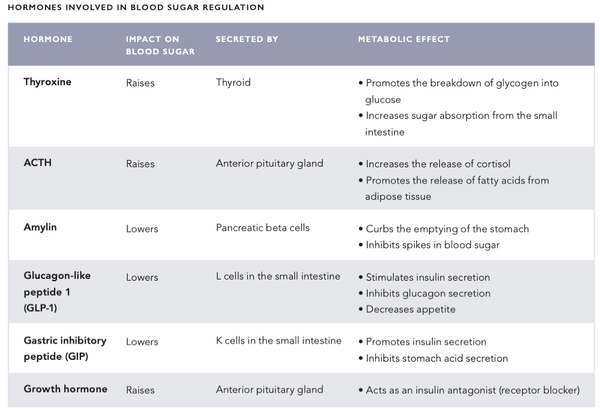
The body's blood sugar regulation mechanism is a very sophisticated system that involves several hormones secreted by internal organs (see the table on the following pages). The blood sugar level is regulated by a negative feedback system – this system seeks to bring the body into a state of systemic homeostasis, i.e. a state of equilibrium.
When the blood sugar level is high, pulsations of hormones such as insulin are secreted into the circulation, lowering the blood sugar level. Conversely, when the blood sugar level is low, glucagon and other hormones are secreted into the circulation to raise the blood sugar level.
Systemic blood sugar level monitoring takes place in the beta cells of the islets of Langerhans in the pancreas as well as in the glucose-sensing neurons of the hypothalamus in the brain.
GLYCOLYSIS
Glycolysis is a simple part of the glucose metabolism in which glucose is broken down into pyruvate or lactate. In addition to energy production, glycolysis regulates the secretion of insulin and is linked to glucose-stimulated insulin secretion in the pancreatic beta cells. In such cases, there is significantly increased secretion of the glucokinase enzyme which breaks down glucose into glucose-6-phosphate. Due to glucokinase activity being strongly linked to blood glucose levels and hence insulin secretion, glucokinase is considered the main blood sugar level sensor.
GLUCONEOGENESIS
Gluconeogenesis is a metabolic process in which glucose is produced from lactic acid, glycerol, alanine, and glutamine. Gluconeogenesis is activated particularly when the diet is lacking in carbohydrates. It also allows the body to stabilize the blood sugar level in the event that it becomes excessively low.
Gluconeogenesis takes place primarily in the liver (alanine) and the renal capsules (glutamine) and, according to the latest studies, also in the intestines (particularly the small intestine).
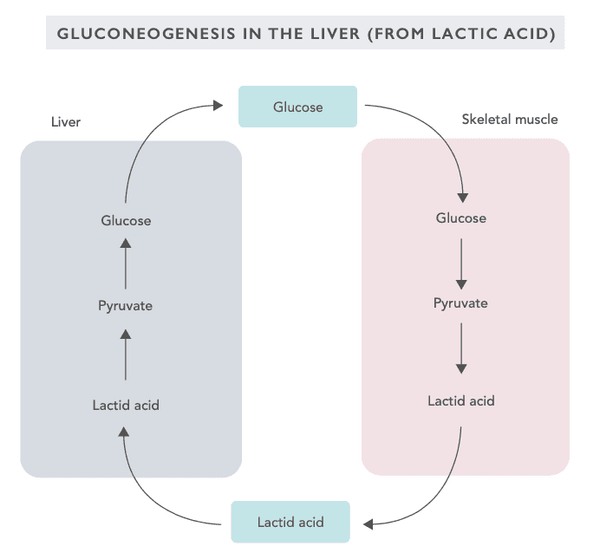
Gluconeogenesis is activated when a person is in a starvation mode, following a low-carbohydrate diet, performing intensive exercise or fasting. Most of the biochemical reactions of gluconeogenesis are reversed compared to those of glycolysis. The enzymatic reactions of gluconeogenesis are regulated by the hormone glucagon secreted by the pancreas.
GLYCOGEN
Glycogen is a large-size molecule formed of several (up to 30,000) glucose molecules. Glycogen is stored in the liver (10 % of the weight), muscle cells (2 % of the weight), and, to a lesser extent, red blood cells. In addition to glucose, glycogen binds triple the amount of water. Because of this, a person’s body weight may fluctuate by several kilograms within a 24-hour period depending on the fill level of the glycogen reserves.
The glycogen storage in the liver acts as an energy reserve for the entire body’s energy production needs, and those of the central nervous system in particular. The glycogen storage in the muscles is only used for the energy production of muscle cells. The amount of glycogen present is determined by physical exercise, the basal metabolic rate, and eating habits.
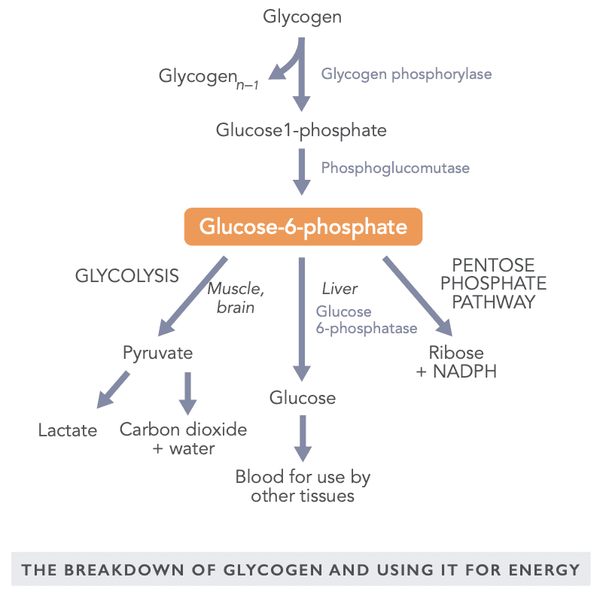
The glycogen reserves are especially important for the regulation of blood sugar between meals and during intensive exercise. Glucose may also be used for energy under anaerobic conditions. Conversely, fatty acids are broken down into energy only under aerobic conditions. The brain needs a steady level of glucose although it is able to utilize, for example, the ketone bodies produced by the liver during fasting.
A metabolically active glycogen breakdown product is glucose 6-phosphate in which the glucose molecule binds with one phosphate group. It may be used for energy in a muscle under either aerobic or anaerobic conditions, utilized via the liver as glucose elsewhere in the body or converted into ribose and NADPH for use in various tissues (for example in the adrenal gland, red blood cells, mammary glands, and the fat cells in the liver).
FOODS AND SUPPLEMENTS THAT HELP REGULATE BLOOD SUGAR LEVELS
The effects of various foods on blood sugar have conventionally been described using the glycemic index (GI). It represents the change in blood sugar caused by the food compared to a reference value (glucose solution). Conversely, the glycemic load (GL) indicates the total effect the meal has on blood sugar. The concepts of GI and GL were originally developed for diabetics. There have also been attempts to apply them to the treatment of other health problems (such as cancer and heart diseases) with conflicting results. The GI and GL have been criticized for not considering individual variation and insulin response triggered by food. Also, the glycemic response triggered by a meal consisting of various ingredients cannot be reliably estimated.
However, an insulin index has been developed to best represent the effect of food on the secretion of insulin. So far the insulin index has received little attention although it appears to be of use to type 1 diabetic who has previously been accustomed to estimating and counting the number of dietary carbohydrates. The satiety score can be used along with the insulin index. It represents the sensation of satiety yielded by a specific food. For example, potatoes have a high insulin index of 121, however, their satiety score is also very high at 323 (see the table on the following pages for more information).
A low GI diet is often recommended for individuals focused on weight loss. However, the glycemic index alone appears to have little impact on weight loss compared to other diets of the same calorie content.
According to studies, a diet with a consistently high glycemic load is associated with a higher level of silent inflammation. Conversely, the Mediterranean diet with a low glycemic load is quite effective in reducing obesity, insulin resistance, high blood pressure, and high cholesterol, at least in women suffering from metabolic syndrome according to one study.
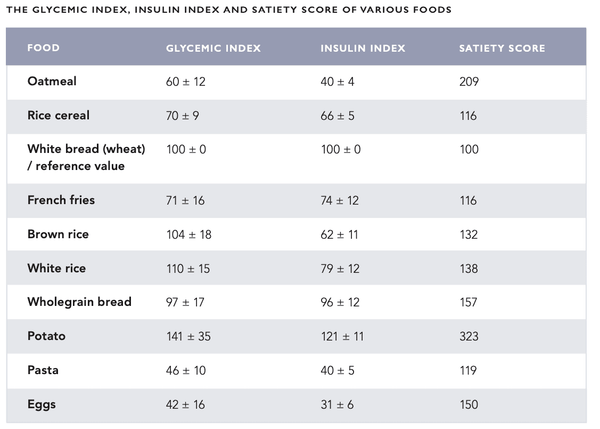
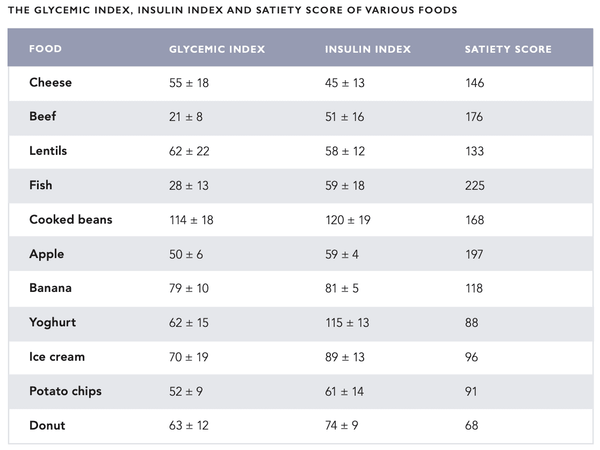
A diet of foods with a fairly low glycemic load and insulin index represents the preferred option for both health and mental alertness. Foods with a high insulin index should be consumed after exercise to replenish the glycogen reserves in the muscles and liver with insulin.
The spike in blood sugar caused by a meal may be balanced with various foods and supplements (see the following chapters). In Western countries, cinnamon is typically added to sweet desserts and baked goods that significantly raise blood sugar levels. In China, medicinal fungi and herbs
are included in the diet due to their blood sugar balancing properties. The Ayurvedic medicine of India incorporates several herbs to balance blood sugar, such as Gymnema Sylvestre. The Hindi word ”gurmar” means “sugar destroyer”.
REGULATING BLOOD SUGAR WITH FOOD
Cinnamon is a spice derived from plants of the genus Cinnamomum. Cinnamon sold in Europe is usually Chinese (cassia) cinnamon (Cinnamomum cassia, Cinnamomum aromaticum) that contains coumarin which is toxic to the liver and kidneys when consumed in large quantities (see the Nutrition chapter of the Biohacker’s Handbook for more information).
There are numerous varieties of cinnamon in the world with significant variations in the coumarin content. Consuming cinnamon as tea is a traditional way to use it. This method also significantly reduces coumarin intake. The toxicity of coumarin varies between individuals. It is linked to the ability of the genetic variant CYP2A6 to act as part of the liver’s cytochrome P450 system.
The properties of cinnamon that help regulate blood sugar and insulin sensitivity are strongly linked to its high chromium content as well as the effects of polyphenols and volatile polymers. Depending on the study design and dose used, cinnamon lowers the fasting blood sugar levels by 10–29 %. A typical recommended daily amount is 1–6 grams. When deciding on the amount to use, it is recommended you consider your genetic background (CYP2A6 polymorphism), goals, and any potential interactions with medicinal products.
The effects of many performance-boosting herbs and so-called smart drugs are often also associated with blood sugar regulation. For example, according to a meta-analysis from 2014, ginseng root has been found to help lower fasting blood sugar levels.
Foods and spices that help balance blood sugar:
- Cinnamon
- Bilberry
- Garlic
- Sour cherry
- Apple cider vinegar
- Coffee
- Chia
- Caraway
- Ginger
- Shiitake mushroom
- Lemon
- Turmeric
- Cacao (and dark chocolate)
Supplements and remedies that help balance blood sugar:
- Chromium
- Vitamin D
- Alpha-lipoic acid
- Reishi
- Maitake
- Chaga
- Cordyceps
- Psyllium
- MCT oil
- Gymnema sylvestre
- Bitter melon
- Prickly pear cactus
- Fenugreek
- Purslane
- Banaba leaf
- Milk thistle
- Resveratrol
- Magnesium
- Panax ginseng
- Berberine
- Green tea
- Coriander
- Vanadyl sulfate
BLOOD SUGAR BALANCING COCKTAIL
To be consumed approx. 30 minutes before a high-carbohydrate meal:
- 100–300 mg alpha-lipoic acid
- 50–100 mg chromium
- 500–1000 mg berberine or 100-200 mg dihydroberberine
- 200–400 mg EGCG (green tea extract)
- 50 mg resveratrol
- 100 mg magnesium malate
THE EFFECT OF COFFEE ON BLOOD SUGAR REGULATION
Long-term studies have found a link between coffee consumption and higher insulin sensitivity as well as a lowered risk of developing type 2 diabetes (see section “Coffee” in the Nutrition chapter of the Biohacker’s Handbook for more information). Despite these findings, coffee may acutely decrease insulin sensitivity and raise blood sugar levels in individuals unaccustomed to caffeine.
The changes in blood sugar regulation caused by coffee are most probably due to caffeine. Decaffeinated coffee has not been found to cause a similar swing in blood sugar levels. It appears that regular coffee consumption also reduces the effects of coffee on blood sugar.
Individuals with a point mutation in the CYP1A2 gene (variant 164A>C) break down caffeine significantly slower compared to the general population. This is also linked to the blood sugar swings caused by coffee as well as higher levels of fasting blood sugar, particularly in individuals with high blood pressure.
For most people, reasonable coffee consumption (3–4 cups per day) is compatible with a healthy, balanced diet and an active lifestyle. According to a comprehensive meta-analysis, reasonable coffee consumption may extend the life span, lower the risk of developing type 2 diabetes and cardiovascular diseases and prevent premature death from these illnesses.The health benefits of coffee are most likely due to the anti-oxidants it contains, such as polyphenols. Indeed, more than 1,000 antioxidant compounds have been found in coffee, even more than in green tea and cocoa.

SLEEP AND BLOOD SUGAR REGULATION
Blood sugar levels fall steadily when fasting during waking hours. Conversely, blood sugar levels usually remain constant during sleep. This is due to the blood sugar level initially rising by approximately 20 % at the beginning of the sleep cycle and then slowly falling to a normal level. These observations indicate that the utilization of glucose for energy is decreased during sleep. During non-REM sleep, the glucose metabolism of the brain decreases by 11 %. Conversely, glucose metabolism is increased during REM sleep and when awake.

Sleep deprivation has a significant impact on blood sugar regulation. Being severely deprived of sleep (four hours of sleep per night) for as little as one week impairs the use of glucose for energy and raises fasting blood sugar. It is also a predisposing factor for sugar metabolism disorders (pre-diabetes). According to a study, individuals who sleep less than 6.5 hours per night have up to 40 % lower insulin sensitivity compared to those who get a normal amount (7–8 hours) of sleep per night. Impaired insulin sensitivity is a predisposing factor for blood sugar swings, obesity and type 2 diabetes. According to a meta-analysis published in 2015, for blood sugar regulation and diabetes prevention, the optimal amount of daily sleep is 7–8 hours. It is worth noting that excess sleep may also impair blood sugar regulation.
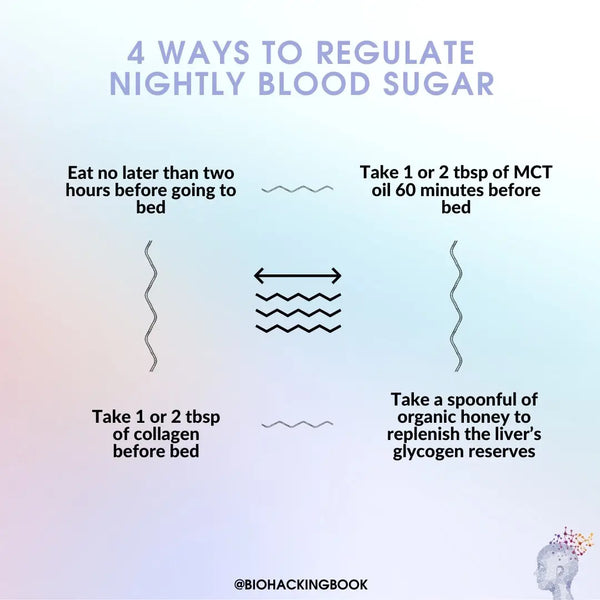
Sleep deprivation also interferes with the sensation of hunger by affecting the secretion of leptin and ghrelin. According to a study conducted on young men, insufficient sleep (4 hours) on just two consecutive nights decreased the level of the satiety hormone leptin by 18 % and increased the level of the hunger hormone ghrelin by 28 %. Individuals experiencing sleep deprivation reported a significant (24 %) increase in appetite, particularly for sweet, salty or starchy foods. More comprehensive population studies have also yielded comparable results.
Read a comprehensive article on optimizing deep sleep and improving sleep quality here.
BALANCE YOUR BLOOD SUGAR LEVELS EASILY WITH INTERMITTENT FASTING
Intermittent fasting means fasting for a significant portion of the day (for example 16 hours) and consuming the daily food intake during the remaining eating window (for example 8 hours). The simplest way to implement this is to extend the overnight fast by skipping breakfast and enjoying the first meal of the day in the afternoon.
The concept of intermittent fasting counters the trend of “six small meals a day” promoted by the food industry and fitness culture of today. Grazing is often rationalized with claims of activating the metabolism and making weight management easier. However, no scientific basis has been found for such claims. In fact, the basal metabolic rate (BMR) increases slightly after a 36-hour fast – it is only after 72 hours that the BMR begins to slowly decrease.
Intermittent fasting is helpful in achieving the desired calorie restriction when the goal is removing waste products from the body (autophagy) or weight loss. Improvements in the regulation of sugar metabolism have also been reported in conjunction with intermittent fasting.
According to one study, there were no significant differences in the energy expenditure of individuals who ate frequently (6 times per day) compared to those who ate more infrequently (2 times per day). The same study found that those who fasted in the morning naturally ate slightly less overall and also consumed a slightly smaller amount of carbohydrates.
From an evolutionary perspective, human beings evolved to eat when there is food available (usually in the evening) – the rest of the time was spent acquiring the food (in the morning and during the day).
In practice, intermittent fasting works well as it allows for the consumption of satisfying meals during the eating window while maintaining a moderate overall energy intake. For example, the consumption of food (particularly carbohydrates) in the evening significantly reduces the levels of stress hormones and promotes sleep as well as stabilizing the secretion of leptin, ghrelin and adiponectin (burning fat). Consuming meals later in the evening also activates the parasympathetic nervous system, calming the body and making it easier to fall asleep.
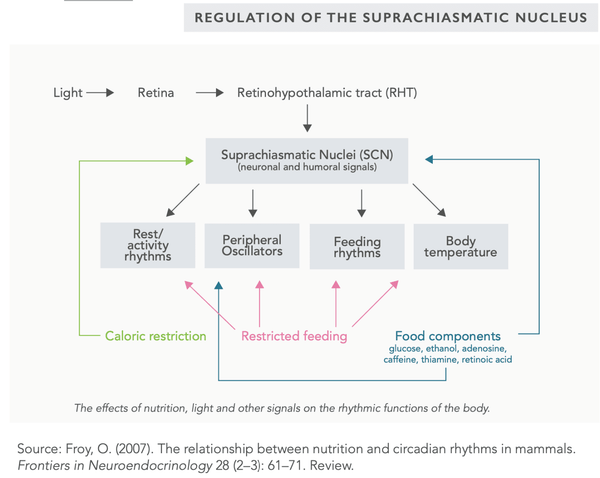
Intermittent fasting (and calorie restriction) may also be used to balance the function of the suprachiasmatic nucleus (SCN) that regulates the circadian rhythm of the body. The primary factor in SCN regulation is light (particularly sunlight).
Water, tea, coffee and mineral water are often consumed to maintain fluid balance when fasting. Low-energy green juices are also a good option as they contain essential micronutrients (see recipes on the following page). Highly active or athletic people may also consume essential amino acids (EAA) in tablet or powder form to maximize recovery.
Intermittent fasting (or fasting in general) is not generally recommended for individuals who are under 18 years of age, pregnant, breastfeeding, severely fatigued or suffering from the chronic fatigue syndrome.
There are various methods of intermittent fasting:
- Fasting for 24 hours 1–2 times per week (”Eat Stop Eat”)
- Fasting for 20 hours followed by a 4-hour eating window (”The Warrior Diet”)
- Fasting for 36 hours followed by a 12-hour eating window (”The Alternate Day Fast”)
- Fasting for 16 hours followed by an 8-hour eating window (”Leangains”)
- Fasting for 18 hours (fatty coffee allowed) followed by a 6-hour eating window (”Bulletproof Intermittent Fasting”)
Health benefits of fasting/intermittent fasting include:
- May extend lifespan by slowing down the aging process
- May reduce the risk of developing metabolic and chronic diseases such as
- Cancer
- Dabetes
- Metabolic syndrome
- Arthritis
- Neurodegenerative diseases (such as Alzheimer's disease)
- May improve insulin sensitivity and lower blood pressure
- May reduce oxidative stress in the body
- May improve the hormonal balance of the body
THE BIOHACKER’S GUIDE TO INTERMITTENT FASTING
Each of the authors of the Biohacker’s Handbook has his own pattern for intermittent fasting that has evolved over various experiments.
Here is a sample pattern for a person who is physically active and works at the office:
- Fasting overnight and delaying the first meal as much as possible (usually until sometime between 3 PM and 6 PM depending on the timing of the previous meal)
- While fasting, drink plenty of fluids such as mineral water (rich in minerals, delays hunger)
- Hunger may be further delayed before the first meal by consuming an apple which is rich in fiber and relatively low energy (< 50 kcal) – consuming a cucumber may also delay hunger (negligible calorie intake)
- Consider the following low-carbohydrate drink recipes below (image)
- The first meal should consist mainly of protein, fibrous vegetables and fat, and a small amount of carbohydrates (if desired)
- The second (and last) meal, consumed between 8 PM and 11 PM, should include plenty of carbohydrates as well as suitable amounts of fat and protein – when consumed close to bedtime it can help optimize sleep
- Physical exercise is often timed either at the end of the fasting period in the afternoon or after the first meal later in the evening

Lifestyle choices that improve insulin sensitivity include:
- Getting sufficient sleep
-
Regular exercise (particularly strength training and high intensity training)
- Combining aerobic exercise and strength training is the most effective way to improve insulin sensitivity
- Stress management and reducing stress
- Weight loss (particularly around the waistline)
- Plentiful intake of soluble fibers from food
- Eating more colorful vegetables, berries and fruits (particularly dark berries such as blueberries and blackcurrants)
-
Using herbs and spices in cooking
-
Turmeric
-
Ginger
-
Cinnamon
- Garlic
- Ginseng
- Fenugreek
- Medicinal fungi (reishi, shiitake, maitake, chaga, cordyceps)
- Apple cider vinegar
- Green tea
-
Reducing the intake of carbohydrates, particularly sugars
-
Regular fasting and intermittent fasting
-
Avoiding excessive sitting
-
Eliminating processed vegetable oils and trans fats from the diet
-
Certain dietary supplements may improve insulin sensitivity:
- Chromium
- Magnesium
- Berberine
- Resveratrol
- Alpha-lipoic acid
MEASURING AND MONITORING BLOOD SUGAR LEVELS
A constant blood sugar level is one of the key factors for the maintenance of good performance levels and mental alert- ness. For diabetics, monitoring blood sugar levels is vital for health. Monitoring one’s own blood sugar may also be useful for people who are not diabetic – such as curious biohackers who are keen to find out what factors in nutrition and lifestyle have an effect on performance and mental alertness.
Blood sugar test strips were introduced in the 1970s. They were soon followed by devices that gave a blood sugar value using the test strips. Today, it is possible to take a quick fingertip sample and have a device analyze it almost instantaneously. Peripheral devices and applications designed for measuring blood sugar are also available for smartphones.
Diabetics might now use monitors placed under the skin that take nearly continuous blood sugar measurements (e.g. every five minutes). These monitors warn the user when the results are too low or too high or when sudden swings in blood sugar are detected. It is possible to collect long-term blood sugar data using such devices.
The latest advancement is a device that uses laser (mid-wavelength infrared) to measure blood sugar without requiring a needle prick. According to a study conducted by the developers, the accuracy of the device is 84 % compared to a blood test. Various nanotechnology-based devices are also in development.
Blood sugar measuring methods under development:
- Contact lenses that measure blood sugar levels
- Infrared and ultrasound devices
- Raman spectroscopy
- Single-use nanobiosensors (blood sugar analysis from saliva) and implants (blood sugar analysis from blood)
- Affinity sensors that take measurements optically
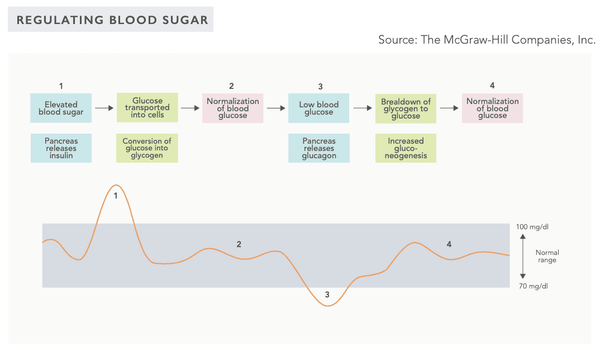
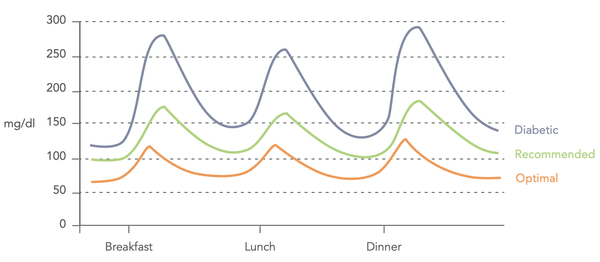 Image: Variations in blood sugar levels (Biohacker's Handbook, 2019).
Image: Variations in blood sugar levels (Biohacker's Handbook, 2019).
INTERPRETING BLOOD SUGAR MEASUREMENTS
The following recommended values are based on several studies. They can be used to determine optimal estimates of the lowest possible risk of developing diabetes, cardiovascular diseases, stroke, metabolic syndrome, etc.
The immediately preceding food intake should be taken into consideration when measuring blood sugar values. For example, individuals who follow a low-carbohydrate diet usually have a lowered tolerance of sugar which may cause high measurement values after a high-carbohydrate meal. This should be taken into consideration when conducting an oral glucose challenge test in a laboratory setting. The recommended adjustment period is four days before the test, during which a minimum of 150 g of carbohydrates per day should be consumed.
-
Fasting blood sugar (fasting plasma glucose, FPG)
- Normal: 4–6 mmol/L or 72–108 mg/dL
- Optimal: 4.0–5.3 mmol/L or 72–95 mg/dL (Life Extension Foundation recommends 4.0–4.7 mmol/L or 72–85 mg/dL)
- Glycated hemoglobin (HbA1C)
- Normal: 20–42 mmol/L / 4.0 % – 6.0 %
- Optimal: 20–34 mmol/L / 4.0 % – 5.3 %
- Disruptive factors may include anemia (excessively low result) or dehydration (excessively high result)
- Oral glucose tolerance test (OGTT)
- Values taken: fasting blood sugar and blood sugar
- 1 hour and 2 hours after the glucose challenge (75 g)
- Normal value at 1 hour: < 10.0 mmol/L or 180 mg/dL Optimal value: < 7.8 mmol/L or 140 mg/dL
- Normal value at 2 hours: < 8.6 mmol/L or 155 mg/dL Optimal value: < 6.7 mmol/L or 121 mg/dL
- Measuring blood sugar levels at 1 hour and 2 hours after a meal
- Self-monitoring blood sugar levels during a 24-hour period (note that the margin of error when using a monitor intended for home use is approximately 10 %)
- Fasting blood sugar after fasting for 12 hours
- Blood sugar immediately before lunch
- Blood sugar one hour after lunch (optimal value < 7.8 mmol/L) or 140 mg/dL
- Blood sugar 2 hours after lunch (optimal value < 6.7 mmol/L) or 121 mg/dL
- Blood sugar 3 hours after lunch (optimal value < 5.3 mmol/L) or 95 mg/dL
If you want to learn how to interpret your own blood sugar levels and, above all, what you can do to balance your blood sugar levels, we recommend taking the Optimize Your Lab Results online course. Below is additional material from the course e.g. for measuring insulin and assessing insulin sensitivity.
FASTING INSULIN
Insulin is a hormone produced and secreted by the beta cells in the pancreas. The body secretes insulin as a response to elevated blood glucose levels that are caused by eating or the secretion of cortisol due to increased stress. Insulin increases glucose absorption and storage and the synthesis of fatty acids and proteins while inhibiting the breakdown of proteins and fatty acids. High insulin levels occur in connection with insulin resistance, obesity, metabolic syndrome, insulinoma, Cushing’s syndrome or excessive insulin or corticosteroid doses. Low insulin levels occur in connection with diabetes, hypopituitarism and various pancreatic illnesses. Ideally, the fasting insulin level should be at the lower end of the reference range.
Insulin inhibits lipolysis, or the breakdown of fat into energy. If the body's stored insulin levels are consistently elevated, the fatty acids circulating in the blood are stored in the adipose tissue. This is called lipogenesis. In particular, the secretion of insulin is stimulated by high blood sugar levels and a carbohydrate-rich diet. Abundant protein intake also increases insulin secretion. Due to these factors, the fasting insulin level should be quite low, in the lower half of the reference range. High insulin levels may also be a predisposing factor for the chronic diseases discussed in the description of the HbA1C value.
- Reference range: 2.0–20 mIU/L
HOMA-IR (INSULIN SENSITIVITY)
By calculating the interaction between fasting glucose and fasting insulin, the beta cell function of the pancreas and the insulin sensitivity of the body may be assessed. Insulin sensitivity depends on how much insulin needs to be secreted to store a certain amount of glucose. Insulin sensitivity is present when only a small amount of insulin is needed to store a certain amount of glucose in cells. Conversely, insulin resistance is present when more insulin is required to store an equivalent amount of glucose.
Insulin resistance and low insulin sensitivity may lead to type 2 diabetes, non-alcoholic fatty liver disease (NAFLD), cardiovascular disease, the accumulation of harmful visceral fat, or even cancer. Normal or optimal insulin sensitivity is generally associated with better health and metabolism.
///






