NAD+ is a coenzyme found in all living cells and is necessary for the basic functioning of the body. NAD+ levels decline as we age, which is thought to be a contributor to the aging process.
Nicotinamide adenine dinucleotide (NAD+/NADH)
NAD was first discovered during yeast fermentation. Since its discovery, it has been found that NAD (nicotinamide adenine dinucleotide) is a major cofactor that partakes in virtually all cellular reactions. These include DNA repair, immune system function, ATP production and circadian clock function. NAD+ promotes energy production and enables cells to work properly. Dysregulation of the NAD+ levels has been associated with metabolic diseases and aging-related diseases, including neurodegeneration, defective immune responses, and cancer.
NAD has two forms – NAD+ and NADH, which both command electron transfer reactions:
- NAD+ is an oxidizing agent that picks up electrons from other molecules and thus becomes reduced
- NADH is a reducing agent that forms from reduced NAD+ and is then used to donate electrons to other molecules, thus becoming NAD+ again
- Electrons of NADH can store energy which gets converted into ATP in the mitochondria during a process called oxidative phosphorylation in the mitochondria (see Biohacker’s Handbook’s Exercise chapter for more information)
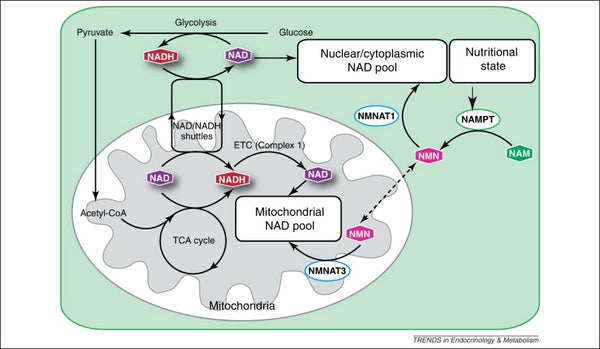
Image: Maintenance of the mitochondrial NAD pool.
Source: Stein, L. & Imai, S. (2012). The dynamic regulation of NAD metabolism in mitochondria. Trends in Endocrinology and Metabolism 23 (9): 420–428.
Introduction to NAD+
Ideally, NAD+ is in a homeostatic status of biosynthesis, consumption, recycling and degradation at both cellular and systemic levels. Human cells can synthesize NAD+ de novo from tryptophan by the kynurenine pathway or from nicotinic acid (NA) by the Preiss‐Handler pathway. However, most NAD+ is recycled from nicotinamide (NAM), NA, nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) in the salvage pathway to maintain the cellular NAD+ levels. NAD+ can be reduced into NADH in various metabolic processes, including glycolysis, fatty acid oxidation and the Krebs cycle.
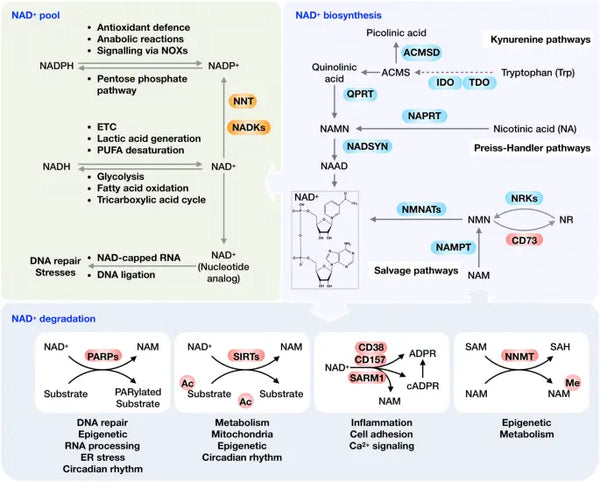
Image: Overview of the NAD+ metabolism and its physiological function.
Source: Xie, N. et al. (2020). NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. Signal Transduction and Targeted Therapy 5 (1): 1–37.
As a co-substrate important to various fundamental macromolecules, NAD+ can be cleaved by NAD+-consuming enzymes. These include PARPs, sirtuins, CD38 and SARM1 to generate NAM and ADP-ribose (see image). Under normal homeostatic conditions, CD38 is expressed at low levels, whereas rising expression of CD38 with aging plays a vital role in age-associated NAD+ reduction. This notion is confirmed by the observation that PARP1 and CD38 inhibition effectively increases total NAD+ availability, leading to SIRT1 activation.
A decrease in NAD+ levels, based on recent science, is associated with aging. It is common that NAD+ levels drop to less than half after the age of 60 compared to levels in your twenties. The big question is why NAD+ levels decline in the first place. There have been a couple of theories on this one, but the latest and most supported theory is that NAD+ levels decline with age because it is being destroyed by the overactivity of a NAD-consuming enzyme CD38. Low NAD+ status is also known to inhibit the body’s immune system and natural defense mechanisms.
CD38, also known as cyclic ADP ribose hydrolase is a glycoprotein found on the surface of many immune cells (white cells in particular) including B lymphocytes, natural killer cells, CD4⁺ and CD8. Usually, more inflammation results in higher CD38 expression, which then depletes NAD+. Therefore, controlling low-grade inflammation and inflammation in general, is the best way to minimize NAD+ loss due to aging.
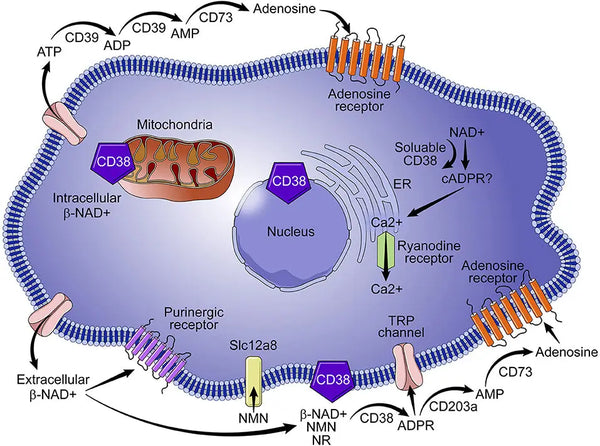
Image: Role of CD38 in NAD+ metabolism.
Source: Hogan, K. & Chini, C. & Chini, E. (2019). The multi-faceted ecto-enzyme CD38: roles in immunomodulation, cancer, aging, and metabolic diseases. Frontiers in Immunology 10: 1187.
NAD+ alleviates oxidative damage caused by viral and bacterial infections
Viral infections and infections in general, cause oxidative stress in host cells (e.g. human cells). Hence, oxidative stress is considered a pathogenic factor in viral infections. The increased cellular reactive oxygen species by viral infection causes for example DNA damage, gene mutation, cell death, viral DNA integration and tumorigenesis. To repair the oxidative stress-induced DNA damage, a large amount of NAD+ is needed and consumed by elevated PARPs in response to virus infection.
Sirtuins are another class of NAD+ consuming enzymes, which have broad-range antiviral properties on diverse viruses (including HIV-1, HCMB, H1N1 and HCV). CD38 is the third NAD+ consuming enzyme that is overexpressed in response to multiple viral infections. CD38 deficiency results in an increased predisposition to several pathogens.
Bacterial infections also induce rapid production of intracellular reactive oxygen species (ROS) either by NADPH oxidases (NOXs) or mitochondria that are essential for macrophages to clear out bacteria. NAD+/NADH exerts the bactericidal activity by promoting the ROS generation, the pro-inflammatory response and the anti-infection autophagy. This beneficial and natural metabolic process needs well-functioning NAD+ metabolism and optimal levels of NAD+ to function properly. Elimination of the ROS results in defective bactericidal (bacteria-killing) activity, allowing bacteria to survive and repeatedly colonize various tissue sites.
This is the reason why using too many antioxidants all the time is not beneficial for the immune system. The key here for optimal homeostasis is to have a balance between oxidative stress and antioxidant capacity.
Emerging evidence supports the hypothesis that the CD38 and products controlled by the CD38/NAD+ axis may play significant roles in the pathogenesis of SARS-CoV-2 infection. Over expression of CD38 in COVID-19 causes cell death mainly by depletion of NAD+. Oral administration of NAD+ precursors (NR, NAM, and NMN) seems to be the most effective approach to replenishing NAD+ levels (see later). Of these NAD+ precursors, NR (supplemented 1 gram per day) has anti-inflammatory effects in different disease conditions. Current scientific evidence seems to confirm that key events of the biosynthesis and consumption of NAD+ play significant roles in the antiviral immune response. Increasing NAD+ levels by modulating the biosynthetic pathways or by reducing NAD+ consumption may help control the hyperimmune response to SARS-CoV-2 infection.
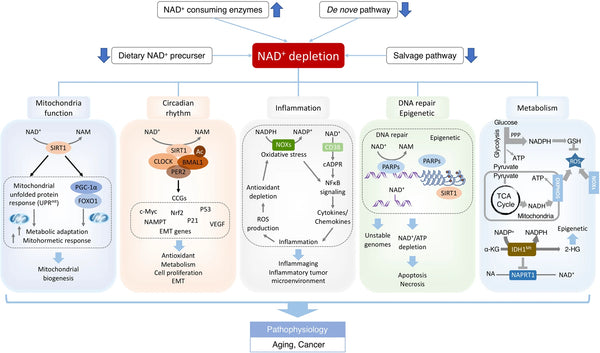
Image: NAD+ deficits in aging-associated dysfunction and cancer.
Source: Xie, N. et al. (2020). NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. Signal Transduction and Targeted Therapy 5 (1): 1–37.
Lifestyle factors that decrease NAD+ levels:
- Circadian rhythm mismatches
- Chronic inflammation and oxidative stress
- Constant caloric surplus (eating too much all the time) – higher NADH, lower NAD+
- Elevated blood sugar and insulin levels
- Chronic alcohol use
Possible consequences of NAD+ deficiency:
- Impaired immune system function
- Accelerated aging
- Mitochondrial dysfunction
- Disturbed circadian clocks (with aging)
- Increased carcinogenesis and risk for cancer
- Increased risk for insulin resistance and the development of diabetes
- Increased risk for obesity
- Increased risk for non-alcoholic fatty liver disease
- Increased risk for neurodegenerative disorders
- Increased risk for heart & kidney failure
Best ways to increase NAD+ levels in the body:
In general, intracellular NAD+ levels are maintained between 0.2 and 0.5 mM, depending on the cell type or tissue. However, the concentration and distribution of NAD+ can fluctuate in response to diverse physiological stimuli and cellular stresses.
- Practice regular (intermittent) fasting and caloric restriction (read more here)
- Activate ketogenesis in the body and have regular glucose restriction periods
- Exercise regularly
- Practice heat alteration (read more here)
- Best food sources of NAD+ precursors include:
- Raw and fermented dairy (high in NR)
- Fatty fish such as salmon, sardines, trout and mackerel (high in niacin)
- Reindeer, beef and chicken liver (high in niacin)
- Pork and turkey (high in tryptophan and niacin)
- Beef (high in niacin)
- Supplement with NAD+ precursors
- Nicotinamide riboside (NR): optimal dose 300 mg per day
- Nicotinamide mononucleotide (NMN): optimal dose 250–500 mg per day
- Liposomal dose is about 10x times smaller
- Use with trimethyl glycine (TMG) for optimal methylation process
- Niacinamide (NAM): optimal dose 250–500 mg per day
- Nicotinic acid (NA): optimal dose 250–500 mg per da
There is no long-term data on the safety of continuous supplementation with NAD+ precursors, NR and NMN in particular. Having too high NAD+ levels may also cause disruptions in the NAD+/NADH homeostasis. These include circadian rhythm mismatches and based on mice studies, sleep cycle disruptions as well as deranged hunger and appetite patterns. Based on this, it may be ideal to take NAD+ precursors in the morning.
Recommended supplements:
Physiological benefits of having high NAD+ in the body:
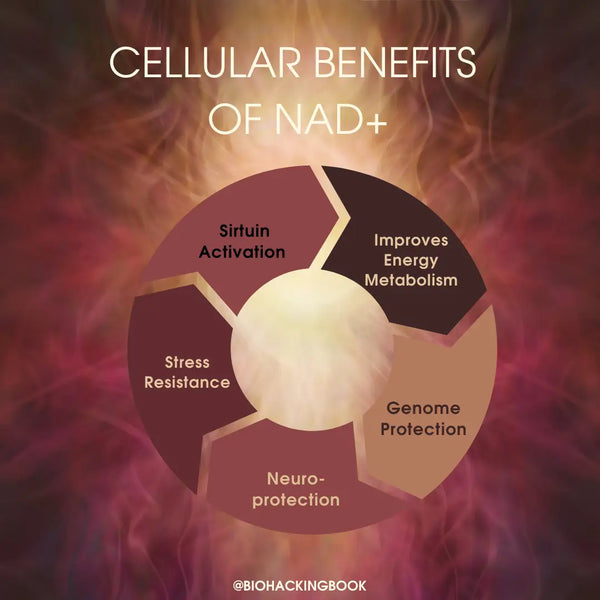
- Optimal mitochondrial functioning
- Crucial biochemical element in energy production and muscle function
- Optimal brain function and prevention of neurodegeneration
- Optimal sleep-wake cycle and maintenance of natural circadian rhythms
- Protection from oxidative stress and inflammation
- Cellular survival and oxygen production
- Slowed aging and longer lifespan & healthspan via mitophagy and DNA repair (based on mice studies)
- Decreased risk of heart disease via reversing age-related arterial dysfunction and improved overall cardiac health (based on mice studies)
- Decreased aging of the skin
///






