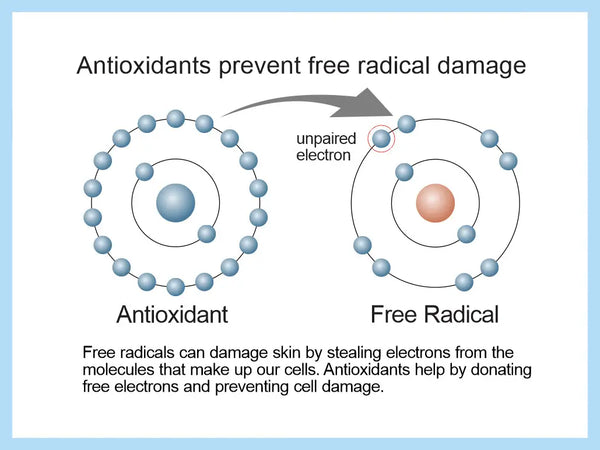
Oxidative stress is cell-level stress
Oxidative stress is a natural biochemical phenomenon in the body, which when overexpressed, can lead to an imbalance in the cellular level that extends throughout the whole body. Oxidation stress can be viewed through a redox reaction (reduction-oxidation). It is a chemical reaction in which one or more electrons are transferred, in whole or in part, from one atom to another. In such a reaction, the electron-donating agent is oxidized and the electron-accepting agent is reduced.
In practice, oxidative stress means that the cells are exposed to too much of the oxidation reaction. This imbalance is caused by the presence of too many oxidizing factors in the body or by a decrease in the body's antioxidant capacity, i.e. the reserve of reducing factors. Ideally, there is a balance between these that supports natural homeostasis.
Under oxidative stress, the amount of reactive and free oxygen radicals increases (increased number of reactive oxygen species or ROS). The molecules containing oxygen atoms possess an odd and free electron, which makes them very unstable and short-lived. The formation of free oxygen radicals in the body is normal, but these become harmful to health in large quantities. Prolonged oxidative stress increases cell death, which in extreme cases can lead to necrosis in tissues.
Oxygen radicals are produced in the body in mitochondrial energy metabolism (cellular respiration), hepatic cytochrome P450 enzymes, and many other cellular oxidative events. External sources of oxygen radicals include air pollution, radiation, smoke, many drugs such as chemotherapy and xenobiotics, or other substances that are foreign to the body. Oxidative stress is also caused by cytokines in various inflammatory conditions and, for example, bacterial infections. The most common reactive oxygen species are superoxide anion (O2−), peroxides such as hydrogen peroxide (H2O2), hydroxyl radical (OH), alkoxide radicals (RO), peroxide radicals (RO) and peroxynitrite (ONOO-) (see image below).
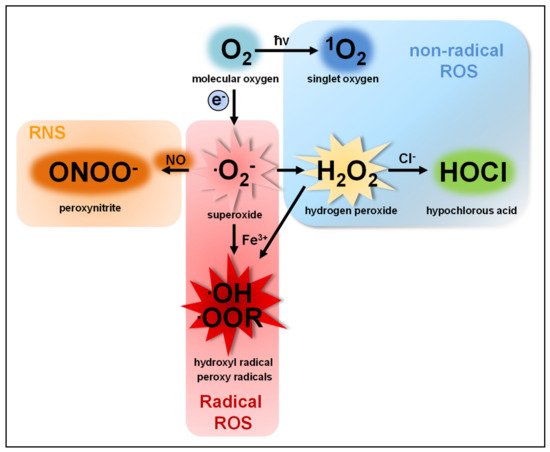
Image source: Herb, M., & Schramm, M. (2021). Functions of ROS in macrophages and antimicrobial immunity. Antioxidants 10 (2): 313.
Free oxygen radical overload has been linked to many different diseases due to their adverse effects at the cellular level (lipid peroxidation, i.e. fat rancidity, protein damage, and DNA damage). Oxidative stress plays a significant role in the development of coronary heart disease, depression, various autoimmune diseases, infections, cancer and many neurodegenerative diseases such as Parkinson's disease, among others. Prolonged excessive oxidative stress is also associated with fatigue and persistent fatigue.
Oxidative stress and telomere shortening
A telomere is a DNA sequence at the end of all chromosomes. Its function is to protect the chromosome and cells against, among other things, oxidative stress and degeneration. Each eukaryotic cell has 46 chromosomes and a total of 92 telomeres at their ends. Telomeres take care of all cell division and DNA information is copied to the new cell. It is also known that the telomere always shortens slightly with each division – they can divide about 50–70 times, after which the cells are no longer able to divide but die (the so-called Hayflick limit).
Elizabeth Blackburn, a Nobel laureate and Australian doctor of molecular biology who has studied telomeres for decades, has found in her research that long-term stress accelerates the shortening of telomeres. Psychological stress appears to increase oxidative stress at the cellular level. In an extensive study published in 2010, Blackburn found that meditation can slow aging. Low levels of oxidative stress and higher levels of telomerase enzyme activity, which prevent telomere shortening, have been observed in long-term meditators.
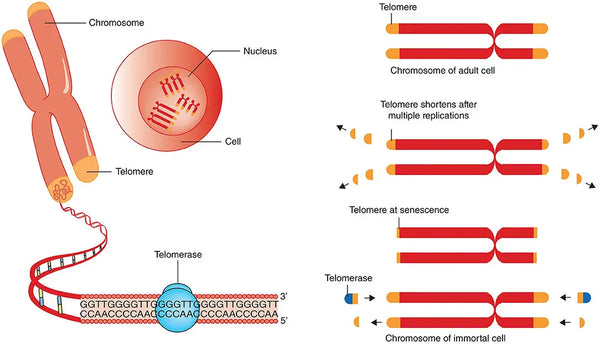
Image: Telomere attrition, telomere length and telomerase.
Source: Vaiserman, A., & Krasnienkov, D. (2021). Telomere length as a marker of biological age: state-of-the-art, open issues, and future perspectives. Frontiers in Genetics 11: 1816.
However, free oxygen radicals are not just a threat to health. In certain situations, they also protect against various infections and thus act as part of the immune system. Short-term oxidative stress may also protect the body from aging due to mitohormesis.
MITOHORMESIS
Mitohormesis is a term used to define a biological response where mitochondrial stress leads to an increase in health and viability within a cell, tissue, or whole organism. The mitochondrial stress response activated by a potentially damaging stimulus requires a coordinated dialogue with the cellular nucleus (mitonuclear communication). This cooperation induced by the hormetic response in mitochondria relies on a variety of signals of which the most important are reactive oxygen species (ROS).
Also, mitochondrial metabolites, proteotoxic signals, the mitochondria–cytosol stress response, and the release of mitokines play a significant role in this process. The activation of mitohormesis has been found to increase lifespan in animal models and it also enhances healthspan by improving the function metabolism and the immune system.
Antioxidants in balancing oxidative stress
According to the oxidative stress hypothesis, from the 1950s until well into the 1990s, it was believed that antioxidants were effective in treating almost any disease. Oxidative stress was also thought to be substantially associated with aging. This is called the Free Radical Theory of Aging (FRTA). It has since been better understood that the use of antioxidants alone does not guarantee health and that many diseases are underpinned by oxidative stress as well as other underlying factors.
However, antioxidants have a place in everyone's diet, as excessive oxidative stress and weak antioxidant capacity are harmful to the body. Antioxidants are obtained from food, but the body also has internally produced antioxidants, which are usually sufficient to balance normal oxidative stress.
The most important antioxidants in food are vitamin C, vitamin A (carotenoids such as beta-carotene) and vitamin E. Health-promoting research findings on dietary antioxidants include astaxanthin, lycopene and green tea (especially its epigallocatechin-3-gallate, ECGC).
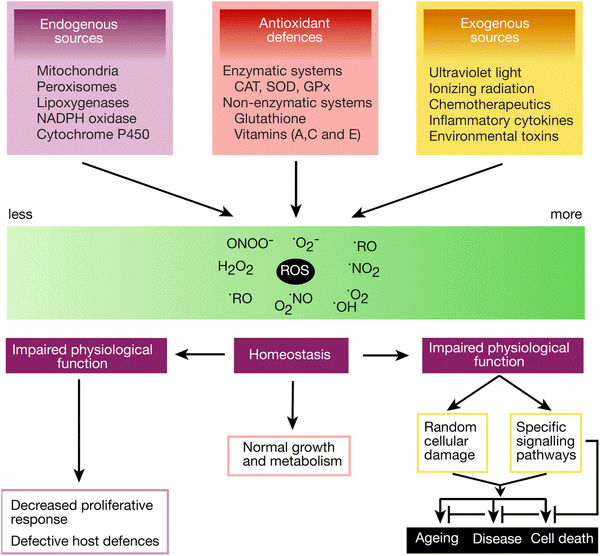
Image source: Krumova, K. & Gonzalo, C. (2016). Overview of Reactive Oxygen Species. Singlet Oxygen: Applications in Biosciences and Nanosciences 1: 1–21. London: Royal Society of Chemistry.
The most important internal antioxidants in the body include superoxide dismutase (SOD), glutathione sulfhydryl (GSH), coenzyme Q10, catalase, and glutathione peroxidase. Peroxydroxins and sulfiredoxin also play an important role. Other important endogenous antioxidants include alpha-lipoic acid, ferritin, urate, bilirubin, metallothionein, L-carnitine, and melatonin.
The relationship between oxidative stress and the body's antioxidant capacity can be accurately measured using a variety of laboratory methods (see later).
ORAC
The so-called ORAC value indicates the antioxidant content of food or a foodstuff. The acronym ORAC comes from the English words Oxygen Radical Absorbance Capacity, which means the ability to reduce free oxygen radicals. The value is obtained, for example, by examining a plant or berry in a test tube (in vitro) and its reaction with a superoxide anion. The ORAC value is thus an indicative figure that does not directly tell us about the antioxidant potential of food in the body.
According to various estimates, the body needs 3,000 to 5,000 ORAC units per day to protect cells from oxidative stress.

In 2012, the USDA withdrew the ORAC value for foods due to its insufficient health evidence. It is therefore unclear whether the ORAC value can be used directly to assess the health benefits of food.
Dietary antioxidants also have a wealth of benefits other than their effect on free oxygen radicals. By combining the properties of berries, vegetables, fruits, spices, and mushrooms such as gourd, protection against oxidative stress can be achieved in support of general health.
Measuring oxidative stress
Oxidative stress is an imbalance between the production of free radicals and the existing antioxidant capacity (also known as redox balance). In general, the decrease in free radical formation is due to increased antioxidant capacity, and a corresponding decrease in antioxidant capacity may be associated with increased production of free oxygen radicals. By determining these concentrations, the balance of oxidation-reduction reactions, i.e. the general oxidative stress state, can be examined in more detail.
The level of oxidative stress can also be assessed by other laboratory studies. The most important studies measuring oxidative stress are presented in the table below.
Table: Laboratory markers describing oxidative stress.
|
Marker |
Action |
Reference range & optimal range |
|
|
|
|
|
|
|
||
|
FRAS test (Free Radical Analytical System) |
|
|
|
///





