Mitochondria are vital organelles located in the cytoplasm of eukaryotic cells. With well-functioning mitochondria, every process in the body, from movement and exercise to digestion and recovery, works better. They play an essential role in energy generation and many other cellular processes. Mitochondria offer the location for oxidative phosphorylation, which produces ATP, the cell’s primary energy source. Structurally, mitochondria can be evaluated through an outer membrane and a highly folded inner membrane that forms compartments necessary for their metabolism. Alongside energy production, mitochondria perform other activities such as calcium signaling and regulation of cellular metabolism in addition to apoptosis (programmed death), which makes them vital for cellular homeostasis maintenance.
The Fundamentals of Mitochondrial Function
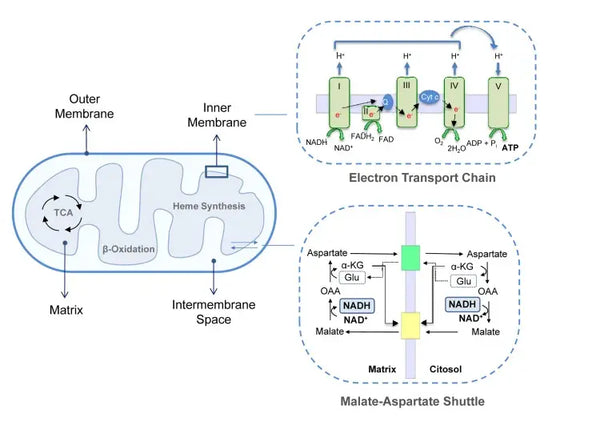
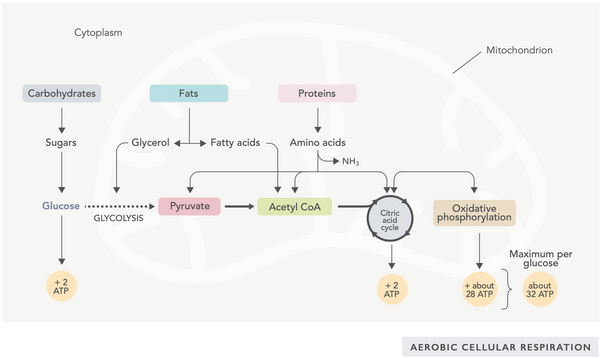
ATP generation (the primary energy molecule used by cells) is the fundamental feature of mitochondrial activity. This process occurs via two main biochemical pathways: the citric acid cycle (TCA cycle or Krebs cycle) and the electron transport chain (ETC).
Citric Acid Cycle
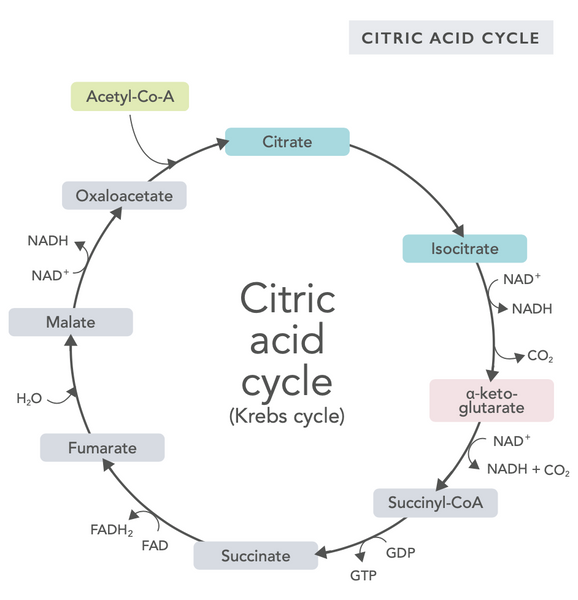
The citric acid cycle, or Krebs cycle (named after the Nobel prize winner Hans Adolf Krebs, who discovered it), occurs in cell mitochondria.(1)The primary metabolic compound of the citric acid cycle is acetic acid (acetyl coenzyme A) produced from fatty acids, carbohydrates and proteins.(2)
The various reactions of the citric acid cycle (see image) form hydrogen ions and electrons which are then transferred to the inner mitochondrial membrane for oxidative phosphorylation (binding energy to ATP molecules through oxidation) and the electron transport chain. The reaction releases NADH and small amounts of ATP and carbon dioxide.
The citric acid cycle involves ten steps, each affected by B vitamins, certain minerals such as magnesium and iron, and the liver’s main antioxidant, glutathione. The reactions are inhibited by heavy metals such as mercury, arsenic and aluminum.
The energy-rich NADH molecules capture most energy generated during the citric acid cycle. For each acetyl coenzyme A molecule, three NADH molecules are generated and then used for energy in the following reaction (oxidative phosphorylation).
The regulation of the citric acid cycle is determined by the availability of various amino acids and feedback inhibition (for example, if too much NADH is produced, several enzymes of the citric acid cycle are inhibited, slowing down reactions).
Oxaloacetate is a compound that fulfills a sudden need to produce energy (for instance, in the brain or muscles). Taking an oxaloacetate supplement may be useful, and it may even boost the regeneration of mitochondria in the brain, reduce silent inflammation in the body, and increase the number of nerve cells.(3)
Simply put, the body incorporates ingenious systems that convert consumed food into electrons, used as energy for various needs.
Oxidative Phosphorylation
Oxidative phosphorylation consists of the electron transport chain and ATP synthase. Oxidative phosphorylation produces the most energy generated in aerobic conditions (ATP). It is a continuation of the citric acid cycle.
In the electron transport chain, hydrogen ions (H+) are released into the mitochondrial intermembrane space. The hydrogen ions released from the intermembrane space move back into the mitochondrion through ATP synthase. Using the energy released in the process, ATP synthase converts the ADP used for energy into ATP again.
Ubiquinone (coenzyme Q10) contributes to the electron transport chain. It has been used for decades as a dietary supplement. Low cellular ubiquinone levels may be a predisposing factor for various illnesses due to insufficient aerobic energy production in the cells. In addition, the use of cholesterol medication (statins) is a contributive factor to ubiquinone deficiency.(4)
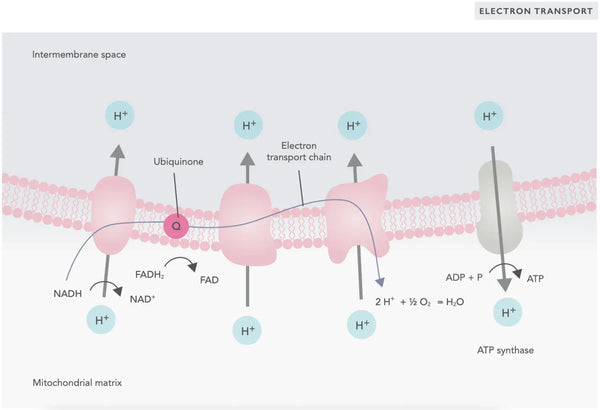
This coordinated set of reactions generates ATP and establishes a proton gradient across the inner mitochondrial membrane, a process known as chemiosmosis. The energy stored in this gradient drives the synthesis of ATP, linking the flow of electrons through the ETC to the generation of cellular energy.(5)
Factors Influencing Mitochondrial Efficiency
Mitochondrial efficiency, which is obviously vital for optimal cellular function, is influenced by various factors. Below are described the most important ones.
Genetic Makeup
Mitochondria possess a separate and individual DNA (mitochondrial DNA, mtDNA) distinct from the nuclear DNA. MtDNA encodes for essential components of the electron transport chain and mitochondrial proteins. Mutations in mtDNA can lead to dysfunctional proteins, disrupting the electron transport chain and ATP synthesis. These may result in reduced energy production and increased reactive oxygen species (ROS) generation, contributing to mitochondrial and cellular dysfunction.(6)
Oxidative Stress
Mitochondria are a significant source of ROS, which are byproducts of oxygen metabolism. While low levels of ROS function in cellular signaling, excessive ROS can cause oxidative damage to mitochondrial proteins, lipids and DNA. This oxidative stress compromises mitochondrial integrity and function, inhibiting ATP production and leading to further ROS production in a deleterious cycle. Antioxidant defenses, including superoxide dismutase and glutathione peroxidase, play a critical role in mitigating this damage.(7-8)
Nutrient Availability
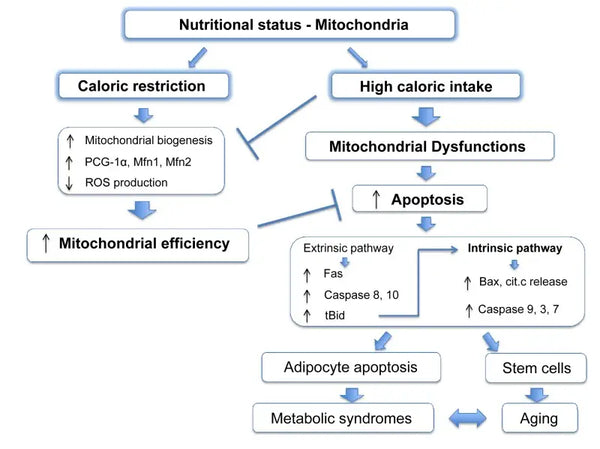
Mitochondria require specific substrates for energy production. Glucose and fatty acids are primary sources for ATP generation via glycolysis and β-oxidation, respectively. The availability of these substrates directly impacts mitochondrial function. For instance, in conditions of nutrient surplus, such as high glucose availability, mitochondria can produce excessive amounts of ATP and ROS, potentially leading to metabolic disturbances.(9-10)
Conversely, nutrient scarcity can limit mitochondrial energy production, affecting cell function and survival. Contrary to some reports from animal studies on the benefits of calorie restriction on mitochondrial biogenesis, it seems that it does not increase mitochondrial biogenesis. However, it preserves mitochondrial function by protecting the integrity and function of existing cellular components.(11-12)
Lifestyle Factors
Exercise
Physical activity influences mitochondrial quantity and quality. Exercise stimulates mitochondrial biogenesis, leading to an increase in mitochondrial density and efficiency in energy production.(13) Especially endurance training enhances the oxidative capacity of mitochondria, improving their ability to utilize oxygen for ATP production (read more in detail later in the article).
Diet
Dietary components significantly affect mitochondrial function. Macronutrient ratios, caloric intake, and specific nutrients (like antioxidants, vitamins and minerals) influence mitochondrial metabolism. Diets rich in nutrients that support mitochondrial function can enhance energy production and reduce oxidative stress (see later). Activating ketosis may also increase mitochondrial biogenesis and improve mitochondrial efficiency.(14-15)
Environmental Factors
Exposure to environmental stressors, such as toxins, pollutants, heavy metals (e.g., lead, mercury, arsenic, and cadmium), and radiation, can adversely affect mitochondrial function. These stressors can induce oxidative damage, disrupt electron transport chain activity, and impair mitochondrial dynamics, including fusion and fission processes.(16)
Technological Interventions
Photobiomodulation (or red light therapy)
Photobiomodulation is the utilization of non-ionizing photonic energy to trigger photochemical changes within cellular structures that are receptive to photons, particularly in mitochondria. Red light and NIR light therapy are believed to work mainly through photo acceptors. Red light waves penetrate the skin and reach the cell’s mitochondria, increasing the cells’ energy production. Various events lead to this, for example, the acceleration of mitochondrial respiration via cytochrome c oxidase.(17) Read about the health benefits of Photobiomodulation here.
Infrared Sauna
Infrared saunas use infrared radiation, which heats body tissues directly instead of air. The frequency of the radiation emitted by infrared saunas is 3–12 μm, which falls under far-infrared (FIR). Far-infrared light has been found to have tissue-level effects, particularly on the mitochondria respiratory chain in the cell energy production process and the blood supply of tissues by dilating blood vessels and improving circulation.(18)
Pulsed Electromagnetic Field (PEMF) Therapy
PEMF therapy uses electromagnetic fields to promote various physiological processes. Research suggests that PEMF can improve mitochondrial function by increasing cellular oxygen consumption and enhancing the production of ATP. PEMF therapy directly affects the mitochondria, returning cells to a healthier energy and electric charge state.(19-20) Read the comprehensive guide on PEMF therapy here.
Hyperbaric Oxygen Therapy (HBOT)
HBOT involves breathing pure oxygen in a pressurized environment. Hyperbaric Oxygen Therapy has been clinically proven to revitalize the mitochondria and increase ATP formation by providing supraphysiologic amounts of oxygen necessary for cellular respiration. A recent study reported that HBO increased mitochondria biogenesis and autophagy by partly increasing the production of reactive oxygen species. This process produced new healthy mitochondria, and old dysfunctional mitochondria were destroyed. This study also found increased activation of mitochondria DNA transcription and replication.(21-22) Learn more about HBOT here.
Nutritional and Supplemental Support for Mitochondrial Function
The role of nutrition in maintaining mitochondrial health is pivotal, with certain nutrients being particularly crucial for optimal mitochondrial function. These include the following listed below.
- Coenzyme Q10 (CoQ10): Ubiquinone is fat-soluble and resembles a vitamin. Ubiquinone functions as an electron carrier in the electron transfer chain in mitochondria (see figure on the right) and furthers the secretion of ATP. Ubiquinone levels decrease in various tissues with aging.(23)
- Magnesium: Involved in ATP synthesis and crucial for maintaining mitochondrial integrity and function.(24)
- B Vitamins: Including B1 (thiamine), B2 (riboflavin), B3 (niacin), B5 (pantothenic acid), B6, B7 (biotin) and B12. These B vitamins are crucial for various aspects of mitochondrial energy metabolism.(25)
- Alpha-lipoic acid: ALA is a powerful antioxidant that also helps regenerate other antioxidants. It has a role in energy metabolism and may improve mitochondrial function.(26)
- Carnitine (specifically Acetyl-L-Carnitine): Transports fatty acids into mitochondria for beta-oxidation, which is crucial for energy production, particularly in muscle cells.(27)
- Creatine: Creatine is a naturally occurring compound in the body that helps supply energy to cells, particularly muscle cells, by increasing the formation of adenosine triphosphate (ATP). Creatine supplementation can enhance mitochondrial efficiency, particularly in high-intensity exercise.(28)
- Pyrroloquinoline Quinone (PQQ): PQQ is an antioxidant that increases mitochondrial biogenesis via PGC-1 protein content and supports mitochondrial function.(29)
- Omega-3 Fatty Acids (EPA and DHA): Omega-3s are essential for maintaining mitochondrial membrane fluidity and function. These are mainly found in fish oil and fatty fish.(30)
- Nicotinamide Riboside (NR) and Nicotinamide Mononucleotide (NMN): These NAD+ precursors are essential for mitochondrial function and energy production. Supplementing with NR or NMN may help raise NAD+ levels and thus improve mitochondrial efficiency.(31) Read more about optimizing NAD+ here.
- Curcumin: Curcumin is the active compound in turmeric. It has been shown to protect mitochondria from oxidative damage and improve their function.(32)
- Selenium: An essential trace element that plays a role in protecting mitochondria from oxidative stress. Selenium also upregulates mitochondrial biogenesis.(33)
Exercise and Mitochondrial Biogenesis
Regular physical activity is a critical factor in promoting mitochondrial biogenesis, which leads to the creation of new mitochondria, thereby enhancing their quantity and functional capacity in cells. Different forms of exercise exert distinct effects on mitochondrial dynamics. The two most important forms or exercise are aerobic training and resistance training.
Aerobic Exercise
Mitochondrial density in skeletal muscle cells increases noticeably during aerobic exercise (e.g., running, cycling and swimming).
Mitochondrial biogenesis is regulated by PGC-1α (peroxisome proliferator-activated receptor gamma coactivator 1-alpha), an essential regulatory protein, which expression aerobic exercise stimulates. PGC-1α coactivates nuclear respiratory factors (NRFs) and mitochondrial transcription factor A (TFAM), which are necessary to transcribe mtDNA and replicate mitochondria. This leads to increased oxidative phosphorylation efficiency, better endurance and greater ATP generation in muscle cells.(34)
High-intensity interval training (HIIT) is particularly effective for increasing the number of mitochondria and the maximal oxygen uptake (VO2max).(35-36)
Resistance Training
Mitochondrial function and efficiency are the primary targets of resistance training (e.g., weight lifting and bodyweight exercises). It triggers changes in mitochondrial protein synthesis, enhancing present mitochondria's quality and effectiveness. Strength training also increases the production of enzymes in electron transport chains and the Krebs cycle, further improving cell capacity for ATP synthesis. It can also increase the number and size of mitochondria in muscle cells, although not to the same degree as aerobic exercise.(37-38)
The combined effect of these exercise modalities on mitochondrial biogenesis and function highlights the importance of physical activity in maintaining and improving mitochondrial health. Regular aerobic and resistance training engagement comprehensively enhances cells' mitochondrial density, efficiency and energy metabolism. These adaptations are crucial for athletic performance and are significant in health maintenance, disease prevention and managing conditions associated with mitochondrial dysfunction.
However, one should note that training for both strength and endurance simultaneously leads to less adaptation, as protein kinases PKB and AMPK block each other's downstream signaling, hindering the concurrent training effect.(39)
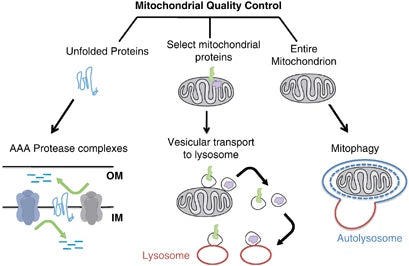
Mitophagy and Cellular Health
Mitophagy refers to a selective type of autophagy that plays an essential role in cellular conditions by being able to degrade only the damaged or nonfunctioning mitochondria. This mechanism is crucial for the health of mitochondria and a cell, preventing mRNAs that encode defective subunit assembly from accruing, thereby saving further crises in cellular functions and numerous pathologies.(40)
Eliminating the damaged mitochondria through mitophagy helps reduce oxidative stress, apoptosis and inflammation (associated with various pathologies). Impaired mitophagy facilitates the accumulation of dysfunctional mitochondria in the pathogenesis of neurodegeneration disorders such as Parkinson's and Alzheimer's disease. Similarly, in metabolic disorders, mitophagy loss leads to mitochondrial metabolism changes, resulting in insulin resistance and type 2 diabetes.(41-42)
It has been shown that caloric restriction promotes mitophagy. This is partly because of the activation of sirtuins (SIRT1), AMP-activated protein kinase (AMPK) and transcription factor EB that support both cellular energy crisis response and initiation of mitophagy. Specifically, sirtuins regulate the function of factors in mitophagy machinery, silencing down damaged mitochondria.(43)
The molecular effects of caloric restriction are mimicked by compounds such as resveratrol (a polyphenol in red wine and some berries) and many other polyphenols. Resveratrol promotes SIRT1 and AMPK activation, increasing mitophagy responsible for mitochondria function improvement and cell health restoration.(44-45)
Mitophagy can be enhanced with intermittent fasting. This improvement is probably mitigated by the metabolic switch from glucose-based to ketone-based energy during fasting and this shift triggers mitophagy.(46-47)
Mitochondrial Dysfunction as a Hallmark of Aging
Understanding how mitochondria contribute to the aging process is at the center of gerontology and cell biology research. As organisms age, mitochondrial dysfunction becomes more common, which plays a role in the physiological side of aging.
The aging mitochondria show decreased production of ATP, which affects the energy-driven processes necessary for cellular well-being. Moreover, the structural changes in mitochondria associated with age interfere even more with this decrease of energetic output, such as abnormal composition of mitochondrial membrane potential and inner-mitochondrial wall integrity.(48)
The position of mitochondrial DNA (mtDNA) near the electron transport chain where reactive oxygen species happens impacts its vulnerability to mutation. However, the mutations build up with time, resulting in mitochondrial dysfunction. Unlike nuclear DNA, which is bound to histones for protection and has a variety of repair mechanisms available, mtDNA lacks protective coating by histones and does not have a wide range of repair methods, so it can easily be damaged.(49)
In cells, mitochondria produce Reactive Oxygen Species (ROS). Although ROS serves as a critical means for the cell to communicate with and adjust itself within its environment, the generation of high levels of these molecules is limited by tight control. Excessive production in various age-related conditions results in cells suffering from oxidative stress. It destroys different cellular components, whether proteins, lipids or DNA. The mitochondria are even the prime recipient of oxidative damage, creating a vicious circle as damaged mitochondria only generate more ROS, thus increasing cellular senescence.(50-52)
Mitochondrial dynamics is crucial for the mitochondrial function that helps to maintain the fusion and fission process. However, these dynamics are disrupted with aging and the mitochondria undergo fragmentation instead of fusion. These changes affect the function of mitochondria and their position in cells.(53)
The loss of mitochondrial function is not a passive aging-related phenomenon but an active participant in the pathogenesis of age-dependent diseases. Mitochondrial dysfunction has been associated with conditions such as neurodegenerative diseases, cardiovascular diseases and metabolic disorders. In these disorders, reduced or defective energy generation, enhanced oxidative stress and the inability to clear damaged mitochondria are essential in disease pathogenesis and course.(54)
Conclusion
Optimization of mitochondrial health and its decline with aging is a challenging task, but luckily there is a lot we can do about it. Principal interventions involve nutritional support of critical substrates for regeneration, including Coenzyme Q10, magnesium, and B vitamins; regular participation in diverse exercise disciplines to augment mitochondrial biogenesis; and lifestyle modifications such as caloric restriction or intermittent fasting to influence mitophagy mechanisms.
Recognizing mitochondrial dysfunction as one of the factors involved in aging makes these strategies essential for supporting cellular health and fighting against age-related degradation. Integrative approaches utilizing the power of scientific-based knowledge in combination with applicable lifestyle alterations can create favorable conditions for prevention and slowing down mitochondrial wear that overall serves an individual's well-being and longevity.
Scientific References
- Leigh, F. (2009). Sir Hans Adolf Krebs (1900–81), pioneer of modern medicine, architect of intermediary metabolism. Journal of Medical Biography 17 (3): 149–154.
- Berg, J. & Tymoczko, J. & Stryer, L. (2002). Biochemistry. 5th edition. Chapter 17, The Citric Acid Cycle. New York: W. H. Freeman.
- Wilkins, H. et al. (2014). Oxaloacetate activates brain mitochondrial biogenesis, enhances the insulin pathway, reduces inflammation and stimulates neurogenesis. Human Molecular Genetics 23 (24): 6528–6541.
- Potgieter, M. & Pretorius, E. & Pepper, M. (2013). Primary and secondary coenzyme Q10 deficiency: the role of therapeutic supplementation. Nutrition Reviews 71 (3): 180–188. Review.
- Saraste, M. (1999). Oxidative phosphorylation at the fin de siecle. Science 283 (5407): 1488-1493.
- Suzuki, T. & Nagao, A. & Suzuki, T. (2011). Human mitochondrial tRNAs: biogenesis, function, structural aspects, and diseases. Annual Review of Genetics 45: 299–329.
- Zhang, Y. & Wong, H. (2021). Are mitochondria the main contributor of reactive oxygen species in cells? Journal of Experimental Biology 224 (5): jeb221606.
- Thannickal, V. & Fanburg, B. (2000). Reactive oxygen species in cell signaling. American Journal of Physiology-Lung Cellular and Molecular Physiology 279 (6): L1005-L1028.
- Marín-García, J. & Marín-García, J. (2013). Mechanisms of bioenergy production in mitochondria. Mitochondria and Their Role in Cardiovascular Disease 99–121.
- Bottje, W. (2019). Oxidative metabolism and efficiency: the delicate balancing act of mitochondria. Poultry science 98 (10): 4223–4230.
- Pintus, F. & Floris, G. & Rufini, A. (2012). Nutrient availability links mitochondria, apoptosis, and obesity. Aging (Albany NY) 4 (11): 734–741.
- Lanza, I. et al. (2012). Chronic caloric restriction preserves mitochondrial function in senescence without increasing mitochondrial biogenesis. Cell Metabolism 16 (6): 777-788.
- Drake, J. & Wilson, R. & Yan, Z. (2016). Molecular mechanisms for mitochondrial adaptation to exercise training in skeletal muscle. The FASEB Journal 30 (1): 13–22.
- Jornayvaz, F. & Shulman, G. I. (2010). Regulation of mitochondrial biogenesis. Essays in Biochemistry 47: 69–84.
- Elamin, M. & Ruskin, D. & Masino, S. & Sacchetti, P. (2017). Ketone-based metabolic therapy: is increased NAD+ a primary mechanism? Frontiers in Molecular Neuroscience 10: 377.
- Fowler, B. (1978). General subcellular effects of lead, mercury, cadmium, and arsenic. Environmental Health Perspectives 22:37-41.
- Hamblin, M. (2018). Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochemistry and Photobiology 94 (2): 199–212.
- Vatansever, F. & Hamblin, M. (2012). Far infrared radiation (FIR): its biological effects and medical applications. Photonics and Lasers in Medicine 4: 255–266.
- Hollenberg, A. & Huber, A. & Smith, C. & Eliseev, R. (2021). Electromagnetic stimulation increases mitochondrial function in osteogenic cells and promotes bone fracture repair. Scientific Reports 11 (1): 19114.
- Tai, Y. et al. (2020). Magnetic fields modulate metabolism and gut microbiome in correlation withPgc-1 alpha expression: Follow-up to an in vitro magnetic mitohormetic study. The FASEB Journal 34 (8): 11143–11167.
- Tezgin, D. & Giardina, C. & Perdrizet, G. & Hightower, L. (2020). The effect of hyperbaric oxygen on mitochondrial and glycolytic energy metabolism: the caloristasis concept. Cell Stress and Chaperones 25 (4): 667-677.
- Chen, W. et al. (2020). Hyperbaric oxygen protects against myocardial ischemia‑reperfusion injury through inhibiting mitochondria dysfunction and autophagy. Molecular Medicine Reports 22 (5): 4254-4264.
- Wang, Y. & Hekimi, S. (2019). The complexity of making ubiquinone. Trends in Endocrinology & Metabolism 30 (12): 929-943.
- Volpe, S. (2013). Magnesium in disease prevention and overall health. Advances in Nutrition 4 (3): 378S-383S.
- Depeint, F. & Bruce, W. & Shangari, N. & Mehta, R. & O’Brien, P. (2006). Mitochondrial function and toxicity: role of the B vitamin family on mitochondrial energy metabolism. Chemico-biological Interactions 163 (1-2): 94-112.
- Solmonson, A. & DeBerardinis, R. (2018). Lipoic acid metabolism and mitochondrial redox regulation. Journal of Biological Chemistry 293 (20): 7522-7530.
- Gnoni, A. & Longo, S. & Gnoni, G. & Giudetti, A. (2020). Carnitine in human muscle bioenergetics: can carnitine supplementation improve physical exercise? Molecules 25 (1): 182.
- Wax, B. et al. (2021). Creatine for exercise and sports performance, with recovery considerations for healthy populations. Nutrients 13 (6): 1915.
- Hwang, P. et al. (2020). Effects of pyrroloquinoline quinone (PQQ) supplementation on aerobic exercise performance and indices of mitochondrial biogenesis in untrained men. Journal of the American College of Nutrition 39 (6): 547-556.
- Jeromson, S. & Hunter, D. (2014). Influencing mitochondrial membrane composition and bioenergetics through omega-3 supplementation. The Journal of Physiology 592 (Pt 9): 1913-1914.
- Elhassan, Y. e al. (2019). Nicotinamide riboside augments the aged human skeletal muscle NAD+ metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Reports 28 (7): 1717-1728.
- de Oliveira, M. et al. (2016). Curcumin, mitochondrial biogenesis, and mitophagy: Exploring recent data and indicating future needs. Biotechnology Advances 34 (5): 813-826.
- Handy, D. & Joseph, J. & Loscalzo, J. (2021). Selenium, a micronutrient that modulates cardiovascular health via redox enzymology. Nutrients 13 (9): 3238.
- Fernandez-Marcos, P. & Auwerx, J. (2011). Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. The American Journal of Clinical Nutrition 93 (4): 884S-890S.
- Helgerud, J. et al. (2007). Aerobic high-intensity intervals improve VO2max more than moderate training. Medicine and Science in Sports and Exercise 39 (4): 665–671.
- Burgomaster, K. et al. (2008). Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. Journal of Physiology 586 (1): 151–160.
- Wilkinson, S. et al. (2008). Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. The Journal of Physiology 586 (15): 3701-3717.
- Porter, C. & Reidy, P. & Bhattarai, N. & Sidossis, L. & Rasmussen, B. (2015). Resistance exercise training alters mitochondrial function in human skeletal muscle. Medicine and Science in Sports and Exercise 47 (9): 1922-1931.
- Baar, K. (2006). Training for endurance and strength: lessons from cell signaling. Medicine & Science in Sports & Exercise 38 (11): 1939-1944.
- Ashrafi, G. & Schwarz, T. (2013). The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death & Differentiation 20 (1): 31-42.
- Green, D. & Galluzzi, L. & Kroemer, G. (2011). Mitochondria and the autophagy–inflammation–cell death axis in organismal aging. Science 333 (6046): 1109-1112.
- Mishra, E. & Thakur, M. (2023). Mitophagy: A promising therapeutic target for neuroprotection during ageing and age‐related diseases. British Journal of Pharmacology 180 (12): 1542-1561.
- Cantó, C., & Auwerx, J. (2011). Calorie restriction: is AMPK a key sensor and effector? Physiology 26 (4): 214-224.
- Um, J. et al. (2010). AMP-activated protein kinase–deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 59 (3): 554-563.
- Das, S. & Mitrovsky, G. & Vasanthi, H. & Das, D. (2014). Antiaging properties of a grape-derived antioxidant are regulated by mitochondrial balance of fusion and fission leading to mitophagy triggered by a signaling network of Sirt1-Sirt3-Foxo3-PINK1-PARKIN. Oxidative Medicine and Cellular Longevity 2014: 345105.
- Longo, V. & Panda, S. (2016). Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metabolism 23 (6): 1048-1059.
- Mehrabani, S. & Bagherniya, M. & Askari, G. & Read, M. I& Sahebkar, A. (2020). The effect of fasting or calorie restriction on mitophagy induction: a literature review. Journal of Cachexia, Sarcopenia and Muscle 11 (6): 1447-1458.
- Wei, Y. & Lee, H. (2002). Oxidative stress, mitochondrial DNA mutation, and impairment of antioxidant enzymes in aging. Experimental Biology and Medicine 227 (9): 671-682.
- Zapico, S. & Ubelaker, D. (2013). mtDNA Mutations and Their Role in Aging, Diseases and Forensic Sciences. Aging and Disease 4 (6): 364-830.
- Hernansanz-Agustín, P. & Enríquez, J. (2021). Generation of reactive oxygen species by mitochondria. Antioxidants 10 (3): 415.
- Dunn, J. & Alvarez, L. & Zhang, X. & Soldati, T. (2015). Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biology 6: 472-485.
- Correia‐Melo, C. et al. (2016). Mitochondria are required for pro‐ageing features of the senescent phenotype. The EMBO Journal 35 (7): 724-742.
- Ong, S. & Kalkhoran, S. & Cabrera-Fuentes, H. & Hausenloy, D. (2015). Mitochondrial fusion and fission proteins as novel therapeutic targets for treating cardiovascular disease. European Journal of Pharmacology 763: 104-114.
- Chen, G. & Kroemer, G. & Kepp, O. (2020). Mitophagy: an emerging role in aging and age-associated diseases. Frontiers in Cell and Developmental Biology 8: 200.