Эта статья глубоко погружается в нейропластичность и ее глубокое влияние на когнитивный рост. Эта статья предлагает действенные способы повышения нейропластичности и адаптивности мозга. Исследуйте когнитивные упражнения, осознанность и сенсорное взаимодействие, чтобы способствовать гибкости мозга. Раскрыть связь между физической активностью, питанием, сна и оптимальной нейропластичностью.
Введение
Нейропластичностьтакже известный как пластичность мозга или нейронная пластичность, относится к способности мозга реорганизоваться, формируя новые нейронные связи и модифицируя существующие. Его также можно назвать процессом, который включает адаптивные структурные и функциональные изменения в мозг. Нейропластичность изменила наше понимание мозга, обеспечивая научную основу для замечательной устойчивости и адаптивности человеческого мозга. (1)
Идея нейропластичности была впервые предложена в начале 20 -го века Сантьяго Рамон и Каджалом, отцом современной нейробиологии. (2) Однако только во второй половине 20 -го века концепция получила широкое признание благодаря достижениям в области исследований нейробиологии и технологий визуализации.
Исследования показали, что мозг постоянно изменяется в ответ на внутренние и внешние стимулы. (3) Каждый опыт, мысль и эмоция могут изменить структуру и функцию нашего мозга. Например, изучение нового навыка, такого как игра на музыкальном инструменте, жонглирование или выступление на новом языке, может привести к новым связям между нейронами. В то же время травматические события могут привести к потере связей.
Механизмы, лежащие в основе нейропластичности, включают изменения в силу и количество связей между нейронами и образование новых нейронов и синапсов. Сложное взаимодействие генетических, эпигенетических факторов и факторов окружающей среды, включая физические упражнения, диету, стресс и социальное взаимодействие, стимулирует эти изменения. (4)
Одним из наиболее захватывающих последствий нейропластичности является то, что ее можно использовать для стимулирования восстановления и реабилитации после травмы или заболевания. Например, у пациентов с инсультом интенсивная реабилитация может способствовать росту новых связей в мозге и улучшить моторную функцию. Точно так же медитация на основе осознанности может уменьшить объем областей мозга, которые обрабатывают болевые сигналы у людей с хронической болью.
Различные типы нейропластичности
Нейропластичность может быть широко разбита на два основных механизмах: Структурная пластичность и функциональная пластичностьПолем Что касается временной шкалы человека, нейропластичность также можно разделить на две фазы - Пластичность развития и взрослый пластичностьПолем Это два аспекта нейропластичности, которые встречаются на разных стадиях жизни.

Структурная пластичность
Структурная пластичность относится к физическим изменениям в мозге, таких как образование или устранение синапсов, рост или ретракция дендритных шипов, а также генерация или потеря нейронов. Считается, что эти изменения лежат в основе способности мозга адаптироваться к новой среде и опыту и особенно важны во время развития, когда мозг быстро растет и меняется. (5)
Функциональная пластичность
Функциональная пластичность, с другой стороны, относится к изменениям функциональных свойств нейронных цепей, таких как изменения в силе синаптических соединений или изменения в схеме активности в нейронных сетях. Эти изменения лежат в основе способности мозга учиться и помнить и адаптироваться к изменению когнитивных требований и условий окружающей среды. (6)
Структурная и функциональная пластичность часто взаимозависимой, с изменениями в одном механизме влияют на другой. Как структурная, так и функциональная пластичность являются критическими компонентами нейропластичности, что позволяет мозгу адаптироваться и изменения в ответ на опыт и стимулы окружающей среды. (7)
Пластичность развития
Пластичность развития и пластичность взрослых являются двумя аспектами нейропластичности, которые встречаются на разных стадиях жизни. Пластичность развития относится к процессу нервной пластичности, который происходит во время развития мозга, от эмбрионального развития до детства и подросткового возраста. В течение этого времени мозг очень податлен и реагирует на опыт, с нейронными связями и цепями формируются и совершенствуются в ответ на сенсорный ввод и стимулы окружающей среды. Пластичность развития играет важную роль в нормальном развитии мозга, включая формирование функциональных нейронных цепей и установление критических когнитивных и поведенческих функций. (8)
Взрослый пластичность
В отличие, взрослый пластичность Относится к способности мозга претерпевать пластические изменения в ответ на опыт или травму во время взрослой жизни. Хотя степень пластичности, как правило, ниже во взрослом возрасте, чем во время развития, в мозге взрослого мозга все еще существует значительная способность к нейронной пластичности.
Взрослый пластичность - это продолжающийся процесс обучения и адаптации, который происходит на протяжении всей жизни. Он играет решающую роль в поддержании когнитивных и поведенческих функций в изменяющихся средах. (9)
Одним из ключевых различий между пластичностью развития и пластичностью взрослых является природа пластических изменений. Во время разработки пластичность часто включает формирование новых синапсов, обрезку неиспользованных связей и рост и реорганизацию дендритных и аксонов. Напротив, пластичность взрослых включает в себя укрепление или ослабление существующих соединений за счет изменений синаптической силы и роста новых соединений посредством образования новых синапсов или прорастания новых дендритных процессов.
Ключевые компоненты нейропластичности
1. Синаптическая пластичность
Синаптическая пластичность относится к способности синапсов, соединений между нейронами, чтобы изменить свою силу в ответ на активность. Это фундаментальный механизм, лежащий в основе обучения и памяти и формирования новых нейронных связей. Синаптическая пластичность может возникать как в возбуждающих, так и в ингибирующих синапсах и обусловлена изменениями в высвобождении нейротрансмиттеров и экспрессии рецепторов на постсинаптической мембране.
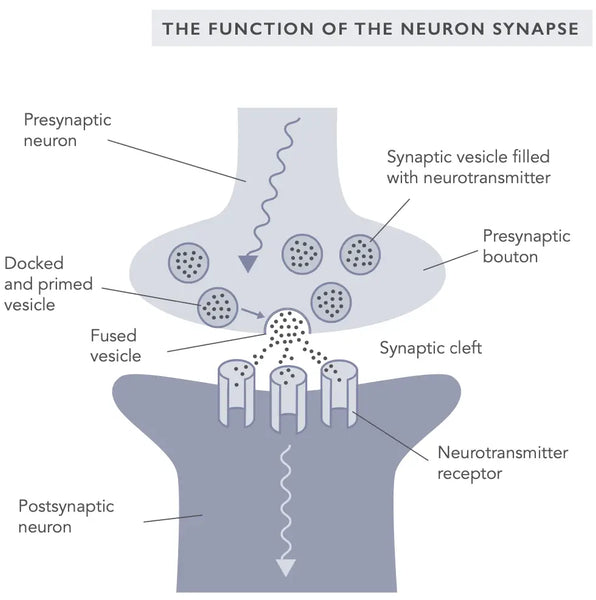
Двумя наиболее хорошо изученными формами синаптической пластичности являются долгосрочное потенцирование (LTP) и долгосрочная депрессия (LTD), также известная как и ебрская пластичность (ссылаясь на нейропсихолога Дональда Хебба, который сначала ввел синаптическую пластичность в 1949 году).(10)
ЛTP - это процесс, с помощью которого сила синапса увеличивается в ответ на повторную активность. Считается, что он лежит в основе укрепления нейронных связей во время обучения и формирования памяти. Ltd, с другой стороны, является процессом, посредством которого сила синапса уменьшается в ответ на низкочастотную или длительную активность. Считается, что Ltd играет роль в ослаблении нейронных связей во время забывания и вымирания. (11) ВедущийEAD больше о LTP, LTD и формировании памяти из Глава Mind's Mind's Mind BioHacker.
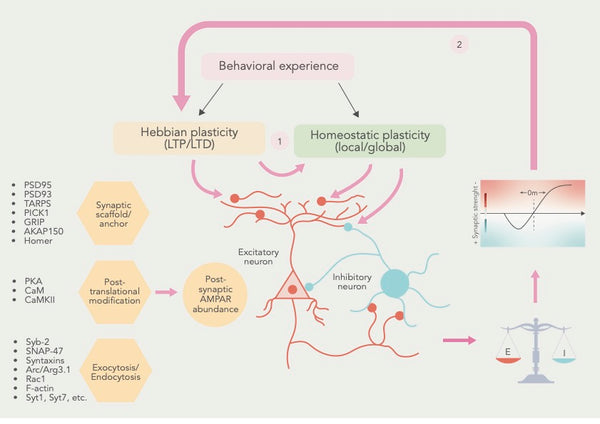
Фигура: Кооперативные отношения между и еббийской и гомеостатической пластичностью.
Источник: Li, J. & Park, E. & Zhong, L. & Chen, L. (2019). Гомеостатическая синаптическая пластичность как механизм метапластичности - молекулярная и клеточная перспектива. Современное мнение о нейробиологии 54: 44–53.
E = синаптическое возбуждение I = синаптическое ингибирование
В дополнение к LTP и LTD были идентифицированы многие другие формы синаптической пластичности, в том числе Метапластичность, что относится к изменениям в пороге для индукции LTP и LTD и гомеостатическая пластичность, что относится к способности нейронов регулировать свою активность в ответ на изменения в сетевой активности. (12)
Собравши эти формы синаптической пластичности, ученые пришли к выводу, что еврбийская и гомеостатическая синаптическая пластичность сходятся на общих клеточных процессах и что гомеостатическая пластичность регулирует состояние синапсов, чтобы воздействовать на ирбийскую пластичность (см. Рисунок выше).
Различные молекулярные и клеточные механизмы регулируют синаптическую пластичность, включая активность протеинкиназ и фосфатаз, синтез и деградацию белков и изменения в экспрессии генов. Эти механизмы чувствительны к различным экологическим и эмпирическим факторам, включая сенсорный ввод, стресс и социальное взаимодействие. (13–15)
Нейрогенез
Нейрогенез - это процесс, с помощью которого в мозге генерируются новые нейроны, особенно в Гиппокамп, регион, важный для обучения и памяти. Это фундаментальный механизм, лежащий в основе способности мозга адаптироваться и реагировать на экологические и опытные факторы.
Нейрогенез возникает в подгранулярной зоне зубчатой извилины гиппокампа, где нервные стволовые клетки вызывают промежуточные клетки -предшественники, которые, в свою очередь, вызывают незрелые нейроны. Эти незрелые нейроны затем мигрируют на слой гранулярных клеток гиппокампа, где они созревают и интегрируются в существующую нейронную схему. Хотя новые нейроны также были обнаружены в других областях, степень нейрогенеза в этих областях, таких как неокортекс и гипоталамус, остается спорной. (16)
Регуляция нейрогенеза представляет собой сложный и динамический процесс, на который влияет различные факторы, включая генетику, эпигенетику и факторы окружающей среды, такие как физические упражнения и стресс. Например, исследования показали, что физические упражнения (особенно аэробные упражнения) могут стимулировать нейрогенез, высвобождая факторы роста, такие как нейротрофический фактор, полученный из мозга (BDNF) и инсулиноподобный фактор роста-1 (IGF-1). И наоборот, было показано, что стресс и хроническое воспаление нарушают нейрогенез через провоспалительный цитокин IL-1β. (17–19)
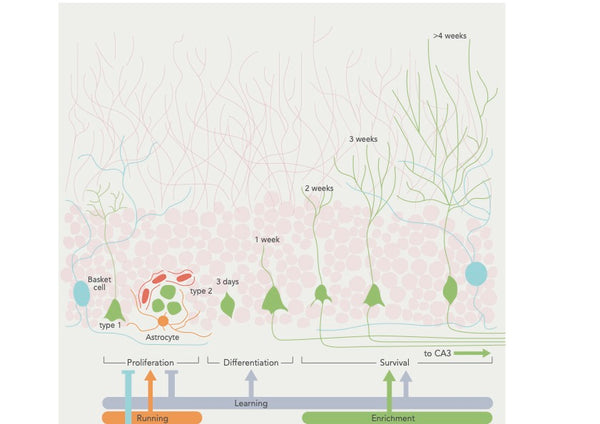
Фигура: Регуляция нейрогенеза поведенческими факторами.
Источник: Aimone, J. et al. (2014). Регуляция и функция нейрогенеза взрослых: от генов до познания. Физиологические обзоры 94 (4): 991–1026.
Функциональная роль нейрогенеза по -прежнему остается предметом активных исследований, но, как полагают, он играет роль в обучении, памяти, регуляции настроения и реакции на стресс. Изучение нейрогенеза имеет важные последствия для разработки новых методов лечения и вмешательств для неврологических и психиатрических состояний. (20–22)
Дендритная арборизация
Дендритная арборизация (или дендритная последствия) относится к процессу, с помощью которого дендриты, разветвленные структуры, которые простираются от тела клеток нейрона, развивают и разрабатывают их разветвления. Этот процесс жизненно важен для установления связности и функциональных свойств нервных цепей в мозге.
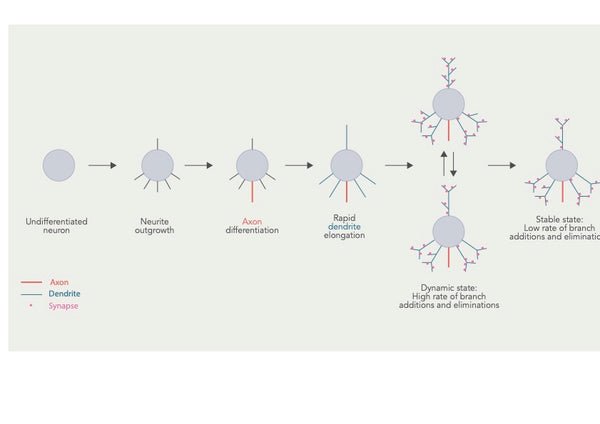 Фигура: Разработка дендритной беседки и несколько перекрывающихся этапов.
Фигура: Разработка дендритной беседки и несколько перекрывающихся этапов.
Источник: Urbanska, M. & Blazejczyk, M. & Jaworski, J. (2008). Молекулярная основа дендритной арборизации. Acta Neurobiologiae Experimentalis 68 (2): 264–288.
Дендритная арборизация является сложным процессом, регулируемым различными факторами, включая генетические и эпигенетические факторы и факторы окружающей среды, такие как сенсорный ввод и нейронная активность. Рост и разветвление дендритов обусловлено активностью сигнальных путей, которые активируются внеклеточными сигналами, такими как факторы роста и нейротрансмиттеры. Эти сигналы могут влиять на экспрессию генов, участвующих в дендритном росте и ветвлении. (23)
Регуляция дендритной арборизации важна для установления и поддержания функциональных нейронных цепей в мозге. Например, дендритная арборизация имеет решающее значение для формирования синапсов, сайтов связи между нейронами. Образцы ветвления дендритов могут влиять на типы и количество образованных синапсов, которые могут глубоко влиять на функциональные свойства нервных цепей. (24)
Дендритная арборизация играет роль в обработке сенсорной информации. Это также важно в когнитивных функциях и формировании памяти. В частности, схемы ветвления дендритов могут влиять на типы и количество образованных синапсов, которые могут глубоко влиять на функциональные свойства нейронных цепей, связанных с обучением и памятью. (25)
Исследования показали, что изменения в дендритной арборизации могут происходить в ответ на учебный опыт, и, как полагают, эти изменения способствуют формированию и поддержанию новых воспоминаний. Например, обучение в задаче пространственной памяти у грызунов увеличило дендритное ветвление в гиппокампе, область мозга, критическая для пространственного обучения и памяти. (26)
Кроме того, исследования показали, что изменения в дендритной арборизации связаны с когнитивным дефицитом при нейродегенеративных заболеваниях. При болезни Альцгеймера дендритные шипы, структуры на дендритах, которые образуют синапсы с другими нейронами, теряются в пораженных областях мозга, что приводит к нарушению синаптической пластичности и когнитивной дефицита. (30)
Миелинизация
Миелинизация является биологическим процессом, в котором аксоны, удлиненные и тонкие клеточные расширения нейронов, которые распространяют электрические импульсы в другие нейроны, подвергаются экранизации, богатым липидом, называемым миелином. Миелиновая оболочка производится олигодендроцитами в центральной нервной системе (ЦНС) и клетках Шванна в периферической нервной системе (PNS). Миелин действует как изолятор, позволяя электрическим сигналам проходить быстрее и эффективно вдоль аксонов. (27)
Процесс миелинизации начинается во время эмбрионального развития и продолжается в раннем взрослом возрасте, с различными областями мозга и нервной системы миелинизируется в разное время. В целом, миелинизация начинается в стволе мозга и спинного мозга и продвигается к коре головного мозга и других более высоких областях мозга. (28) Миелиновые оболочки обычно остаются одинаковой длины в течение длительных периодов времени, предполагая, что в структуре существующего миелина не существует большого количества изменений (см. Изображение ниже). (29)
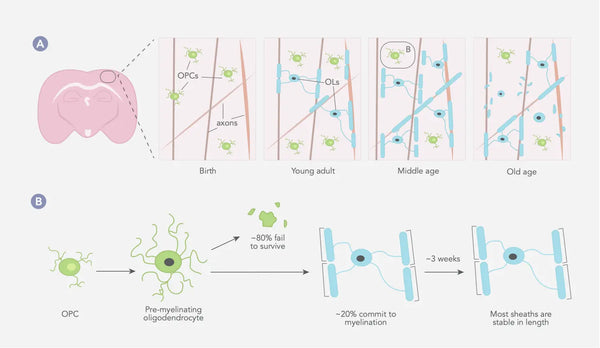
Фигура: Динамика олигодендроцитов и миелина в соматосенсорной коре млекопитающих на протяжении всей жизни.
Источник: Williamson, J. & Lyons, D. (2018). Миелинская динамика на протяжении всей жизни: постоянно меняющийся пейзаж? Границы в клеточной нейробиологии 12: 424.
OPC = клетки -предшественники OPC = OLS = олигодендроциты
Регуляция миелинизации является сложным процессом, на который влияет различные факторы, включая генетику, эпигенетику и факторы окружающей среды, такие как опыт и нейронная активность. Например, исследования показали, что сенсорный опыт может повлиять на время и степень миелинизации мозга. Аналогичным образом, нейронная активность может способствовать миелинизации путем высвобождения сигнальных молекул, таких как BDNF.
В пределах центральной нервной системы процесс миелинизации активируется аксональной активностью и астроцитами, в то время как микроглия/макрофаги ответственны за клиренс миелина. Как только аксоны будут миелинизированы, их постоянное здоровье и функциональность полагаются на предоставление необходимых метаболитов и нейротрофических факторов глиальными клетками. (31)
Функциональная роль миелинизации имеет решающее значение для эффективной и эффективной передачи нервных сигналов в мозге и нервной системе. Миелинизация является ключом к когнитивным и моторным функциям, включая внимание, обучение и координацию. Кроме того, миелинизация также необходима для разработки белого вещества, сети аксонов мозга, которая позволяет различным областям мозга общаться и координировать свою деятельность. (32)
Корковая реорганизация
Кортикальная реорганизация, также известная как корковая пластичностьотносится к способности мозга реорганизовать свои нейронные сети в ответ на изменения в сенсорном вводе или других формах опыта. Этот процесс имеет решающее значение для разработки функциональных нейронных цепей и для способности мозга адаптироваться к изменениям в окружающей среде. Реорганизация коры происходит на нескольких уровнях мозга, от первичных сенсорных областей до областей ассоциации более высокого уровня. (33)
Механизмы, лежащие в основе кортикальной реорганизации, включают изменения в синаптической прочности и связности нейронов. Например, исследования показали, что изменения в сенсорном входе могут привести к изменениям в мощности и количеству синапсов в пораженных областях коры. Точно так же изменения в опыте или поведении могут привести к изменениям в схеме нейронной активности и силе и специфичности синаптических связей. (34)
Функциональные последствия реорганизации коры могут быть полезными или вредными. С одной стороны, реорганизация коры может позволить мозгу адаптироваться к изменениям окружающей среды и восстанавливаться после травмы или заболевания. С другой стороны, реорганизация коры также может способствовать развитию неадаптивных нейронных цепей и хронических боли.
Другой пример в ушах, состояние, когда люди воспринимают звон или другой звук без внешнего стимула. Исследования показали, что реорганизация коры в слуховой коре может играть роль в разработке и поддержании шума в ушах. В частности, мозг может реорганизоваться в ответ на повреждение слуховой системы, что приводит к восприятию фантомных звуков. (35)
Вмешательства, которые способствуют реорганизации коры (например, терапии на основе пластичности головного мозга) могут быть полезны при лечении хронических боли синдромов, инсульта и других форм неврологического повреждения. (36)
Естественные факторы, которые повышают нейропластичность
Было показано, что несколько естественных и технологических методов способствуют нейропластичности и усиливают функцию мозга.
Ниже перечислены лучшие общие факторы образа жизни для улучшения нейропластичности:
- Спать: Адекватный сон (и глубокий сон, в частности,) необходим для функции мозга и было показано, что способствует нейропластичности путем повышения синаптической пластичности и облегчая консолидацию воспоминаний и повышение способности к обучению. (37–38)
- УпражнениеБыло показано, что физические упражнения увеличивают нейропластичность, способствуя образованию новых нейронов, усилению роста дендритных шипов и улучшая функцию существующих нейронных сетей. Показано, что аэробные упражнения увеличивают нейротрофические факторы (BDNF, NGF и GDNF), которые представляют собой белки, которые способствуют росту и выживанию нейронов и глиальных клеток. (39–41)
- МедитацияПоказано, что медитация осознанности способствует нейропластичности, усиливая плотность серого вещества в областях мозга, связанных с вниманием, регуляцией эмоций и самосознанием. Это также может повысить целостность белого вещества, что жизненно важно для связи между различными областями мозга. (42–44)
- Прерывистый пост: Прерывистый пост, который включает в себя ограничение ежедневного потребления пищи до определенных часов, повышает нейропластичность за счет роста новых нейронов и синаптической пластичности. Это также может улучшить когнитивную функцию и снизить риск нейродегенеративных заболеваний. (45)
- Прерывистое метаболическое переключение (IMS): Образ жизни, который включает в себя чередующиеся периоды метаболического стресса и выздоровления, такие как пост и физические упражнения, с последующими питанием, отдыхом и соном, был предложен, повышает функцию мозга и устойчивость. IMS может способствовать здоровью и функции нейрональных цепей, которые поддерживают когнитивные способности и эмоциональное благополучие на протяжении всей жизни. Он широко влияет на множественные сигнальные пути, которые повышают нейропластичность и повышают устойчивость мозга к травмам и заболеваниям. (46)
- Социальное участиеБыло показано, что социальное взаимодействие и взаимодействие способствуют нейропластичности, усиливая рост новых нейронов и улучшая функцию существующих нейронных сетей. Некоторые исследования показали, что социальное взаимодействие может даже защитить от снижения когнитивных средств и возникновения нейродегенеративных заболеваний, таких как болезнь Альцгеймера. Следовательно, поддержание сильной социальной сети и участие в регулярной социальной деятельности может быть эффективным способом поддержки и повышения нейропластичности на протяжении всей жизни (47–48).
- Обогащение окружающей среды: Подход, при котором условия жизни организма оптимизированы для обеспечения разнообразной сенсорной, когнитивной и моторной стимуляции. Было обнаружено, что эта стратегия способствует нейропластичности, индуцируя изменения в нейронной активности и морфологии. В частности, было показано, что обогащение окружающей среды усиливает рост новых нейронов, способствует синаптической пластичности и улучшает функцию существующих нейронных сетей, что приводит к улучшению когнитивных, поведенческих и эмоциональных результатов. (49–50).
- Когнитивная тренировка: Активность, которая бросает вызов мозгу для улучшения нейропластичности мозга. К ним относятся изучение нового языка, воспроизведение музыкального инструмента или решающие головоломки - они могут повысить нейропластичность, способствуя росту и синаптической пластичности новых нейронов. (51–52).
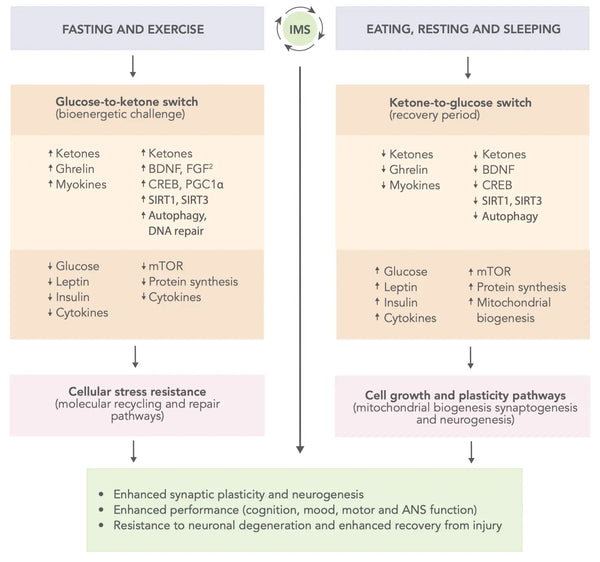
Фигура: Схематическая модель того, как прерывистое метаболическое переключение может оптимизировать производительность мозга и повысить устойчивость к травмам и заболеваниям.
Источник: Mattson, M. & Moehl, K. & Ghena, N. & Schmaedick, M. & Cheng, A. (2018). Прерывистое метаболическое переключение, нейропластичность и здоровье мозга. Nature Reports Neuroscience 19 (2): 81–94.
Питательные факторы, которые поддерживают нейропластичность
- Омега-3 жирные кислотыБыло показано, что жирные кислоты с длинной цепью омега-3, особенно докозагексаеновая кислота (DHA), способствуют нейропластичности, повышая синаптическую пластичность и увеличивая рост дендритных шипов. Они также могут уменьшить воспаление в мозге, что может нарушать нейропластичность. Источники омега-3 включают жирную рыбу, такую как лосось, сардины и добавки. (53–55)
- КуркуминПоказано, что куркумин, соединение, обнаруженное в куркуме, усиливает нейропластичность, способствуя росту новых нейронов и укрепляя синаптическую пластичность. Это также может иметь противовоспалительные эффекты, которые могут улучшить функцию мозга. Интересно, что куркумин также может обратить вспять нарушение познания и пластичность нейронов, вызванную хроническим стрессом. (56–57) - Попробуйте Ageless Defense Dablement, которая содержит B-витамины, полифенолы, аминокислоты и куркумин.
- В витамины B: Было показано, что комплексные витамины и холин улучшают нейропластичность мозга. Они играют решающую роль в различных метаболических путях, которые поддерживают функцию мозга, включая синтезирование нейротрансмиттеров и миелин. Витамины B, особенно витамин B12 и фолат, также участвуют в метилировании ДНК, что может влиять на экспрессию генов при нейропластичности. (58–62)
- Витамин d: Некоторые данные свидетельствуют о том, что витамин D может играть роль в стимулировании нейропластичности. Рецепторы витамина D были обнаружены в различных областях мозга, включая гиппокамп, который участвует в обучении и памяти. Исследования на животных и людях показали, что дефицит витамина D может нарушать когнитивную функцию и уменьшить выработку специфических нейротрофических факторов, необходимых для стимулирования нейропластичности. (63–64) - Получите Ecosh Vitamin K2+D3, чтобы максимизировать нейропластичность.
- ПолифенолыПоказано, что полифенолы улучшают нейропластичность в мозге. Одним из механизмов является их способность модулировать сигнальные пути, участвующие в синаптической пластичности и нейрогенезе. Они также могут оказывать противовоспалительное действие и защищать от окислительного стресса, усиливая функцию нейронов и способствуя нейропластичности. В целом, полифенолы могут предотвратить прогрессирование нейродегенеративных патологий. (65–66) - Попробуйте один из лучших полифенолов: Purovitalis липосомальный кверцетин
Технологические методы, которые поддерживают нейропластичность
- Транскраниальная магнитная стимуляция (TMS): TMS-это неинвазивная техника, которая использует магнитные поля для стимуляции нейронной активности в определенных областях мозга. Было показано, что он усиливает нейропластичность и улучшает когнитивную функцию в различных контекстах, в том числе у людей с депрессией, тревогой и инсультом. (67–69) - - Получите Neorhythm Omnipemf Neurostimulation Device здесь. [См. Изображение ниже]
- Стимуляция мозга: Помимо TMS, другие формы стимуляции мозга, такие как транскраниальная стимуляция непосредственного тока (TDCS) и транскраниальная стимуляция переменного тока (TAC), было показано, что повышают нейропластичность и улучшают когнитивную функцию. (70–71) Узнайте больше о TDCS из Справочник BioHacker.
- Нейрофитак: NeuroFeedback-это метод, который включает в себя мониторинг электрической активности мозга и предоставление обратной связи с человеком в режиме реального времени. Было показано, что он повышает нейропластичность, способствуя росту и синаптической пластичности новых нейронов. Нейрофидбака использовалась для лечения дефицита внимания гиперактивности (СДВГ), тревоги и других неврологических состояний. (72–74). Узнайте больше о нейробиоуправлении от Справочник BioHacker.
- Виртуальная реальность (VR): Виртуальная реальность - это захватывающая технология для обучения, реабилитации и терапевтических целей. В последние годы растущий интерес был использован VR для повышения нейропластичности мозга. VR может улучшить нейропластичность, предоставляя стимулирующую и привлекательную среду, которая бросает вызов мозгу адаптироваться и учиться. Например, VR может имитировать реальные сценарии и предоставлять возможности для обучения и практики в безопасной и контролируемой среде. Это может помочь способствовать росту нового нейронов и повысить синаптическую пластичность. (75–76)
- Программное обеспечение для когнитивного обучения: Программное обеспечение для когнитивного обучения использует компьютерные программы для улучшения когнитивной функции, оспаривая мозг с помощью упражнений на память, задач решения проблем и задач внимания. Кроме того, умственная тренировка может стимулировать высвобождение определенных нейротрансмиттеров, таких как дофамин и ацетилхолин, что может повысить синаптическую пластичность и когнитивную функцию и увеличить BDNF в мозге. (77–79)
Заключение
В заключение, замечательная способность мозга изменить себя посредством различных форм нейропластичности - развития, взрослых, структурных и функциональных - увеличивает мир возможностей для личностного роста и улучшения когнитивных средств. Принятие стратегий этой статьи дает вам инструменты для того, чтобы отправиться в преобразующее путешествие. Вы активно формируете будущее своего мозга, занимаясь умственными упражнениями, развивая осознанность и определяя приоритеты здоровых привычек. Помните, что ключ заключается в последовательности и преданности делу. Итак, справитесь с этим захватывающим приключением открытия потенциала вашего мозга.
Если вы хотите перенести свою игру на следующую октаву, предварительно заказать наше огромное продолжение справочника биогакера « Устойчивое - это книга.
П.с. Эта статья основана на тексте из психической устойчивости, частичной книги.
Ссылки:
- Poderbaugh, M. & Emmady, P. (2022). Нейропластичность. В Statpearls [Интернет]Полем Остров сокровищ (Флорида): Statpearls Publishing.
- Venkataramani, P. (2010). Сантьяго Рамон и Каджал: отец нейронауки. Резонанс 15 (11): 968–976.
- Draganski, B. & Gaser, C. & Busch, V. & Schuierer, G. & Bogdahn, U. & May, A. (2004). Изменения в сером веществе, вызванном обучением. Природа 427 (6972): 311–312.
- Kleim, J. & Jones, T. (2008). Принципы зависимой от опыта нейронной пластичности: последствия для реабилитации после повреждения головного мозга. Журнал речевого языка и исследований слуха 51: S225 - S239.
- Bozelos, P. & Poirazi, P. (2017). Влияние структурной пластичности на емкость памяти. В Перемешивающий мозг (с. 319-341). Кембридж (США): академическая пресса.
- Grafman, J. (2000). Концептуализация функциональной нейропластичности. Журнал расстройств коммуникации 33 (4): 345–356.
- Тауберт, М. и соавт. (2010). Динамические свойства структуры мозга человека: связанные с обучением изменения в областях коры и связанных с ними связей волокна. Журнал нейробиологии 30 (35): 11670–11677.
- Kolb, B. & Gibb, R. (2011). Мозг пластичность и поведение в развивающемся мозге. Журнал Канадской академии детской и подростковой психиатрии 20 (4): 265–276.
- Fuchs, E. & Flügge, G. (2014). Нейропластичность взрослых: более 40 лет исследований. Нейронная пластичность 2014: 541870
- Хебб, Д. (1949). Организация поведения: нейропсихологическая теорияПолем Нью -Йорк: Джон Уайли и сыновья.
- Bliss, T. & Collingridge, G. (1993). Синаптическая модель памяти: долгосрочное потенцирование в гиппокампе. Природа 361 (6407): 31–39.
- Li, J. & Park, E. & Zhong, L. & Chen, L. (2019). Гомеостатическая синаптическая пластичность как механизм метапластичности - молекулярная и клеточная перспектива. Современное мнение о нейробиологии 54: 44–53.
- Magee, J. & Grienberger, C. (2020). Синаптическая пластичность формируется и функции. Ежегодный обзор нейробиологии 43: 95–117.
- Vitureira, N. & de Pasquale, R. & Leão, R. & Rossi, F. (2022). Клеточные и молекулярные механизмы синаптической пластичности при гиппокампальных и корковых синапсах. Границы в клеточной нейробиологии 16: 980623.
- Fox, K. & Stryker, M. (2017). Интеграция хеббийской и гомеостатической пластичности: введение. Философские транзакции Королевского общества B: Биологические науки 372 (1715): 20160413.
- Aimone, J. et al. (2014). Регуляция и функция нейрогенеза взрослых: от генов до познания. Физиологические обзоры 94 (4): 991–1026
- Cotman, C. & Berchtold, N. (2002). Упражнения: поведенческое вмешательство для повышения здоровья мозга и пластичности. Тенденции в нейронауках 25 (6): 295–301.
- Vecchio, L. et al. (2018). Нейропротекторные эффекты физических упражнений: поддержание здорового мозга на протяжении всего старения. Мозговая пластичность 4 (1): 17–52.
-
Saxe, M. et al. (2006). Абляция нейрогенеза гиппокампа нарушает контекстуальную кондиционирование страха и синаптическую пластичность в зубчатой извилине. Труды Национальной академии наук 103 (46): 17501–17506.
- Aimone, J. et al. (2014). Регуляция и функция нейрогенеза взрослых: от генов до познания. Физиологические обзоры 94 (4): 991–1026.
- Ming, G. & Song, H. (2011). Нейрогенез взрослых в мозге млекопитающих: значительные ответы и значительные вопросы. Нейрон 70 (4): 687–702.
- Urbanska, M. & Blazejczyk, M. & Jaworski, J. (2008). Молекулярная основа дендритной арборизации. Acta Neurobiologiae Experimentalis 68 (2): 264–288.
- Cline, H. (2001). Разработка дендритной древесины и синаптогенез. Современное мнение о нейробиологии 11 (1): 118–126.
- Kasai, H. & Fukuda, M. & Watanabe, S. & Hayashi-Takagi, A. & Noguchi, J. (2010). Структурная динамика дендритных шипов в памяти и познаниях. Тенденции в нейронауках 33 (3): 121–129.
- Holtmaat, A. & Svoboda, K. (2009). В зависимости от опыта структурная синаптическая пластичность в мозге млекопитающих. Nature Reports Neuroscience 10 (9): 647–658.
- Demerens, C. et al. (1996). Индукция миелинизации в центральной нервной системе с помощью электрической активности. Труды Национальной академии наук 93 (18): 9887–9892.
- Гарри, Г. и Тьюз, А. (1998). Миелинизация, дисмиелинизация и демиелинизация. Справочник по нейротоксикологии развития 87–115.
- Williamson, J. & Lyons, D. (2018). Миелинская динамика на протяжении всей жизни: постоянно меняющийся пейзаж? Границы в клеточной нейробиологии 12: 424.
- Scheff, S. & Price, D. & Schmitt, F. & Mufson, E. (2006). Синаптическая потеря гиппокампа при ранней болезни Альцгеймера и легкое когнитивное нарушение. Нейробиология старения 27 (10): 1372–1384.
- Nave, K. & Werner, H. (2014). Миелинизация нервной системы: механизмы и функции. Ежегодный обзор биологии клеток и развития 30: 503–533
- Fields, R. (2015). Новый механизм пластичности нервной системы: зависимая от активности миелинизация. Nature Reports Neuroscience 16 (12): 756–767.
- Pascual-Leone, A. & Amedi, A. & Fregni, F. & Merabet, L. (2005). Пластическая кора человеческого мозга. Ежегодные обзоры Нейронауки 28: 377–401.
- Schoups, A. & Vogels, R. & Qian, N. & Orban, G. (2001). Практическая идентификация ориентации улучшает кодирование ориентации в нейронах V1. Природа 412 (6846): 549–553.
- Mühlnickel, W. & Elbert, T. & Taub, E. & Flor, H. (1998). Реорганизация слуховой коры в ушах. Труды Национальной академии наук 95 (17): 10340–10343.
- Merzenich, M. & Van Vleet, T. & Naumum, M. (2014). Терапия на основе пластичности мозга. Границы в нейробиологии человека 8: 385.
- Fattinger, S. et al. (2017). Глубокий сон поддерживает эффективность обучения человеческого мозга. Природная связь 8 (1): 15405.
- Восс, М. и соавт. (2010). Пластичность мозговых сетей в рандомизированном вмешательном исследовании тренировок у пожилых людей. Границы в стареющей нейробиологии 2: 32.
- E Sousa Fernandes, M. et al. (2020). Влияние физических упражнений на нейропластичность и функцию мозга: систематический обзор в исследованиях человека и животных. Нейронная пластичность 2020: 8856621
- Hölzel, B. et al. (2011). Практика внимательности приводит к увеличению региональной плотности серого вещества в мозге. Исследование психиатрии: нейровизуализация 191 (1): 36–43.
- Tang, Y. & Hölzel, B.K. & Posner, M. (2015). Нейронаука медитации осознанности. Nature Reports Neuroscience 16 (4): 213–225.
- Lardone, A. et al. (2018). Медитация осознанности связана с длительными изменениями в функциональной топологии гиппокампа во время состояния покоя: магнитоэнцефалография. Нейронная пластичность 2018: 5340717.
- Brocchi, A. & Rebelos, E. & Dardano, A. & Mantuano, M. & Daniele, G. (2022). Влияние прерывистого голодания на метаболизм мозга. Питательные вещества 14 (6): 1275.
- Mattson, M. & Moehl, K. & Ghena, N. & Schmaedick, M. & Cheng, A. (2018). Прерывистое метаболическое переключение, нейропластичность и здоровье мозга. Nature Reports Neuroscience 19 (2): 81–94.
- Kramer, A. & Bherer, L. & Colcombe, S. & Dong, W. & Greenough, W. (2004). Экологическое влияние на когнитивную и мозговую пластичность во время старения. Журналы серии геронтологии A: Биологические науки и медицинские науки 59 (9): M940 - M957.
- Fratiglioni, L. & Paillard-Borg, S. & Winblad, B. (2004). Активный и социально интегрированный образ жизни в поздней жизни может защитить от деменции. Неврология Лансета 3 (6): 343–353.
- Кемперманн, Г. (2015). Зависимость активности и старение в регуляции нейрогенеза взрослых. Перспективы Cold Spring Harbor в биологии 7 (11): A018929.
- Van Praag, H. & Kempermann, G. & Gage, F. (2000). Нейронные последствия окружающей среды обогащения. Nature Reports Neuroscience 1 (3): 191–198
- Lövdén, M. & Bäckman, L. & Lindenberger, U. & Schaefer, S. & Schmiedek, F. (2010). Теоретическая структура для изучения когнитивной пластичности взрослых. Психологический бюллетень 136 (4): 659–676
- Park, D. & Bischof, G. (2013). Старение ума: нейропластичность в ответ на когнитивные тренировки. Диалоги в клинической нейробиологии 15 (1): 109–119.
- Crupi, R. & Marino, A. & Cuzzocrea, S. (2013). N-3 Жирные кислоты: роль в нейрогенезе и нейропластичности. Текущая лекарственная химия 20 (24): 2953–2963.
- Swanson, D. & Block, R. & Mousa, S.A. (2012). Омега-3 жирные кислоты EPA и DHA: польза для здоровья на протяжении всей жизни. Достижения в области питания 3 (1): 1–7.
- Dyall, S. (2015). Жирные кислоты с длинной цепью омега-3 и мозг: обзор независимых и общих эффектов EPA, DPA и DHA. Границы в стареющей нейробиологии 7: 52.
- Махарджан, Р. и соавт. (2020). Роль образа жизни в нейропластичности и нейрогенезе в стареющем мозге. Cureus 12 (9): E10639.
- Xu, Y. et al. (2009). Куркумин меняет нарушение познания и пластичность нейронов, вызванную хроническим стрессом. Нейрофармакология 57 (4): 463–471.
- Echeverry, M. et al. (2021). Витамины D и B 12, измененная синаптическая пластичность и внеклеточный матрикс. В B-Complex Vitamins-Fources, потребление и новые примененияПолем Intechopen.
- Дауни, Л. и соавт. (2019). Повышенная функциональная связь задних поясной извилины после 6-месячного многовитамина с высокой дозой B-витамином добавки: рандомизированное двойное слепое, плацебо-контролируемое исследование. Границы в питании 6: 156.
- Mattson, M. & Shea, T. (2003). Фолат и метаболизм гомоцистеина при нейронной пластичности и нейродегенеративных расстройствах. Тенденции в нейронауках 26 (3): 137–146.
- Чин, Э. и Го, Э. (2019). Модулируя нейрональную пластичность с холином. Исследование нейронной регенерации 14 (10): 1697.
- Jadavji, N. & Emmerson, J. & Macfarlane, A. & Willmore, W. & Smith, P. (2017). B-витамин и холиновые добавки увеличивают нейропластичность и восстановление после инсульта. Нейробиология болезней 103: 89–100.
- Mayne, P. & Burne, T. (2019). Витамин D в синаптической пластичности, когнитивной функции и нейропсихиатрической болезни. Тенденции в нейронауках 42 (4): 293–306.
- Echeverry, M. et al. (2021). Витамины D и B 12, измененная синаптическая пластичность и внеклеточный матрикс. В B-Complex Vitamins-Fources, потребление и новые примененияПолем Intechopen.
- Vauzour, D. (2012). Пищевые полифенолы как модуляторы функций мозга: биологические действия и молекулярные механизмы, лежащие в основе их полезных эффектов. Окислительная медицина и клеточная долголетие 2012: 914273.
- Figueira, I. & Menezes, R. & Macedo, D. & Costa, I. & Nunes Dos Santos, C. (2017). Полифенолы за барьеры: проблеск в мозг. Текущая нейрофармакология 15 (4): 562–594.
- Халлетт, М. (2007). Транскраниальная магнитная стимуляция: праймер. Нейрон 55 (2): 187–199.
- Jannati, A. & Oberman, L. & Rotenberg, A. & Pascual-Leone, A. (2023). Оценка механизмов пластичности мозга с помощью транскраниальной магнитной стимуляции. Нейропсихофармакология 48 (1): 191–208.
- Auriat, A. & Neva, J. & Peters, S. & Ferris, J. & Boyd, L. (2015). Обзор транскраниальной магнитной стимуляции и мультимодальной нейровизуализации для характеристики нейропластичности после инсульта. Границы в неврологии 6: 226.
- Kricheldorff, J. et al. (2022). Свидетельство нейропластических изменений после транскраниальной магнитной, электрической и глубокой стимуляции мозга. Наук о мозге 12 (7): 929.
- Brunoni, A. et al. (2012). Клинические исследования с транскраниальной стимуляцией постоянного тока (TDCS): проблемы и будущие направления. Стимуляция мозга 5 (3): 175–195.
- Gruzelier, J. (2014). EEG-NEUROFEEDBACK для оптимизации производительности. III: обзор методологических и теоретических соображений. Нейробиологии и биобеводовые обзоры 44: 159–182.
- Trambaiolli, L. & Cassani, R. & Mehler, D. & Falk, T. (2021). Нейрофидбак и стареющий мозг: систематический обзор протоколов обучения для деменции и легких когнитивных нарушений. Границы в стареющей нейробиологии 13: 682683.
- Sitaram, R. et al. (2017). Обучение мозга с замкнутой петлей: наука о нейробиоуправлении. Nature Reports Neuroscience 18 (2): 86–100.
- Laver, K. & George, S. & Thomas, S. & Deutsch, J. & Crotty, M. (2015). Виртуальная реальность для реабилитации инсульта: сокращенная версия Кокрановского обзора. Европейский журнал физической и реабилитационной медицины 51 (4): 497–506.
- Huang, C. et al. (2022). Влияние тренировок моторного контроля на основе виртуальной реальности на воспаление, окислительный стресс, нейропластичность и моторную функцию верхней конечности у пациентов с хроническим инсультом: рандомизированное контролируемое исследование. BMC Неврология 22 (1): 21.
- Anguera, J. et al. (2013). Обучение видеоигр улучшает когнитивный контроль у пожилых людей. Природа 501 (7465): 97–101.
- Lampit, A. et al. (2014). Время глобальных когнитивных выгод от контролируемого компьютерного когнитивного обучения: рандомизированное, активное контролируемое исследование у пожилых людей с множественными факторами риска деменции. А Журнал профилактики болезни Альцгеймера 1 (1): 33–39.
- D'Antonio, J. et al. (2019). Когнитивная тренировка и нейропластичность при легких когнитивных нарушениях (COG-IT): протокол для двух сайте слепого, рандомизированного контролируемого лечения. BMJ Open 9 (8): E028536.







