Изучение устойчивости через призму генетики представляет собой расширяющуюся область интереса, часто пересекающаяся с дисциплинами психологии, нейробиологии и геномики. В то время как концепция устойчивости - способность восстанавливаться или адаптироваться к невзгодам или стрессу - является многогранной и влияющей на сложное взаимодействие генетических, экологических и психологических факторов, были разработаны специфические тесты ДНК, чтобы пролить свет на генетические компоненты этого черта.
Введение
Гены - это сегменты ДНК, которые содержат инструкции, которые необходимо организму, чтобы сделать каждую из многих тысяч белков, необходимых для жизни. Каждый ген состоит из тысяч комбинаций «букв» (называемых основаниями), которые составляют ваш генетический код. Код дает инструкции, чтобы сделать белки, необходимые для правильного развития и функции.(1)
Генетические вариации могут влиять на экспрессию гена, тем самым влияя на метаболические процессы, которые важны для поддержания клеточного здоровья и того, как мы реагируем на экологические вмешательства, такие как диета, образ жизни, добавки и лекарства.
Знание этих генетических вариаций дает непревзойденную информацию о биологических системах, что позволяет практикующим врачам здравоохранения рекомендовать точные вмешательства, направленные на то, чтобы помочь достичь целей и достичь оптимального здоровья.
Понимание генетических маркеров устойчивости
Устойчивость- Способность легко восстанавливаться или приспосабливаться к невзгодам или изменениям - все чаще наблюдается через генетику. Изучая генетические маркеры, исследователи и врачи стремятся раскрыть биологические основы, которые могут объяснить, почему некоторые люди отрываются от стресса и травмы более эффективно, чем другие.(2) Эта растущая область исследований была сосредоточена на тестах ДНК, связанных с устойчивостью, которые изучают генетические маркеры, потенциально ответственные за различия в отдельных реакциях на стресс.
В основе этого исследования лежат эндокринные и нервные системы (В частности, вегетативная нервная система)) В частности, ось гипоталама-гипофизарны (HPA), в частности, является центральной области фокусировки, поскольку она играет критическую роль в регуляции механизма реакции на стресс организма. Эта сложная сеть взаимодействий между гипоталамусом, гипофизом и надпочечниками организует производство и регуляцию ключевых гормонов стресса, таких как кортизол.
Кортизол имеет далеко идущие эффекты на многие функции организма и высвобождается в ответ на стресс и низкую концентрацию глюкозы в крови. При нормальных обстоятельствах кортизол помогает восстановить гомеостаз после стрессовых событий. Однако вариации генов, которые влияют на ось HPA, могут привести к различиям в том, как кортизол производится, регулируется и очищается от тела. Эти генетические вариации могут влиять на физиологическую устойчивость человека - их способность поддерживать или быстро возвращаться к психологическому и физическому здоровью после трудностей.(3)
ДНК -тесты для устойчивости Изучите варианты генов, связанные с рецепторами для стрессовых гормонов, белков, участвующих в путях гормонов стресса и ферментах, которые метаболизируют эти гормоны. Например, вариант гена FKBP5, который участвует в регуляции чувствительности рецептора глюкокортикоидов, посредством которого кортизол оказывает свои эффекты, может изменить реакцию на стресс и потенциально их восприимчивость к расстройствам, связанным с стрессом. Еще дальнейшее расширение масштабов, гены, которые кодируют для нейротрансмиттеров - химические посланники мозга, которые способствуют регуляции настроения и познания - также находятся под центром внимания. К ним относятся гены, такие как COMT, которые играют важную роль в расщеплении дофамина, нейротрансмиттера, связанного с системами удовольствия и вознаграждения.(4)
Кроме того, воспаление это биологический процесс, который был тесно связан со стрессом и расстройствами настроения. Хроническое воспаление, на которое могут влиять генетические вариации, может влиять на функцию мозга и поведение.(5) Таким образом, генетические тесты могут также стремиться идентифицировать варианты в генах провоспалительных цитокинов, таких как IL-6 и TNF, которые могут предрасполагать людей к длительным воспалительным реакциям и влиять на их умственную устойчивость.
Понимание этих генетических маркеров является краеугольным камнем для персонализированных вмешательств. Выявляя генетическую предрасположенность, люди могут предпринять проактивные шаги, чтобы укрепить свою устойчивость благодаря модификациям образа жизни, психологическим стратегиям и медицинским методам, адаптированным к их уникальному генетическому составу. Этот подход иллюстрирует сдвиг в сторону точной медицины, где модель универсального подхода уступает более индивидуальной помощи.
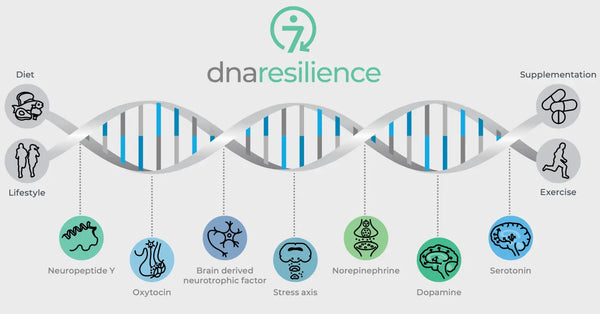
Изображение: Семь молекулярных путей устойчивости.
Типы тестов ДНК для устойчивости
1) Гены реакции на стресс
Гены реакции на стресс являются критическими компонентами системы нашего организма для управления и реагирования на стрессоры. Они кодируют белки, которые регулируют различные физиологические процессы, в том числе высвобождение и действие кортизола, гормона -ключевой в реакции стресса.
CRHR1 (рецептор 1) кортикотропин-рилизинг-гормональный рецептор 1)
CRHR1 кодирует рецептор для кортикотропин-рилизингового гормона (CRH), центрального гормона, инициирующего стресс-реакцию. Когда CRH связывается с CRHR1, он стимулирует выработку и высвобождение адренокортикотропного гормона (АКТГ), который побуждает надпочечников продуцировать кортизол. Варианты в гене CRHR1 могут влиять на то, насколько чувствителен этот рецептор к CRH, что может изменить общую реакцию на стресс. Например, некоторые полиморфизмы могут привести к повышенной реакции на стресс, что может способствовать тревоге или депрессии. Напротив, другие могут ослабить ответ, влияя на способность обрабатывать острый стресс.(6-7)
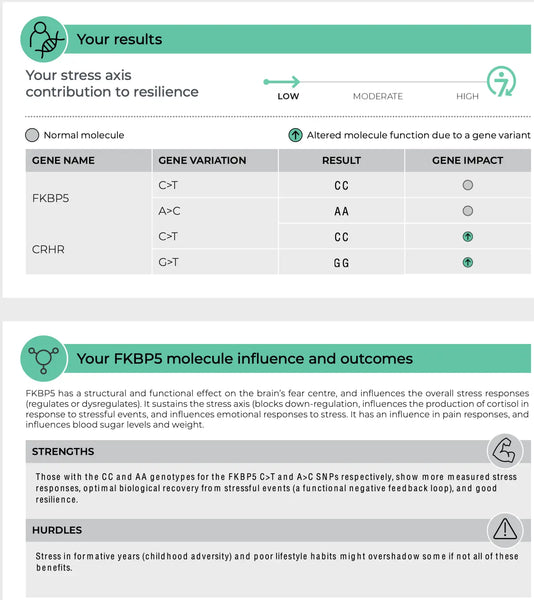
FKBP5 (FK506 -связывающий белок 5)
Ген FKBP5 играет роль в регулировании чувствительности рецептора глюкокортикоидов, который является рецептором, посредством которого кортизол оказывает свои эффекты. Специфические полиморфизмы в FKBP5 были связаны с измененным ингибированием оси HPA и дифференциальными реакциями на глюкокортикоиды. Например, некоторые варианты FKBP5 могут снизить аффинность глюкокортикоидного рецептора к кортизолу, что потенциально приводит к нарушению реакции на стресс и повышенному риску связанных с стрессом психических расстройств, таких как ПТСР или основная депрессия.(8-9)
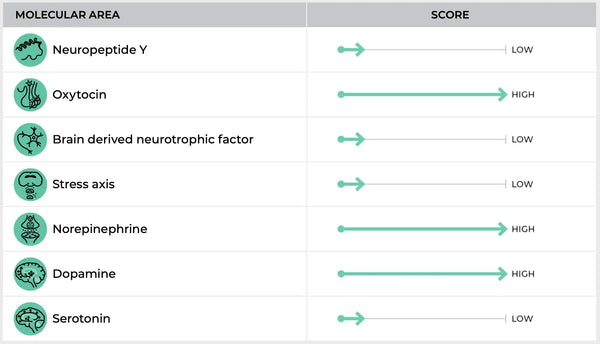
Изображение: Пример страницы Тест ДНК устойчивости.
NR3C1 (подсемейство 3 ядерного рецептора 3, группа C, член 1)
NR3C1 кодирует сам глюкокортикоидный рецептор. Вариации в этом геном могут повлиять на то, как организм реагирует на кортизол. Специфические полиморфизмы в NR3C1 могут влиять на чувствительность или плотность глюкокортикоидных рецепторов, влияя на то, как эффективно кортизол может выполнять свои функции. Это может привести к ряду ответов, от приглушенной реакции на стресс, которая может помешать человеку адекватно реагировать на стрессоры на преувеличенный ответ, который может привести к хроническим симптомам стресса и даже депрессии.(10-12)
Результаты испытаний генов на стресс могут дать представление о предрасположенности человека к проблемам со здоровьем, связанными с стрессом. Например:
-
Повышенная реакция на стресс: Люди с определенными вариантами могут быть более реактивными на стресс и испытывать более высокие уровни кортизола во время стрессовых событий.
-
Сниженная реакция на стресс: И наоборот, у некоторых людей может быть притупленная реакция на стресс, которая может быть защитной в острых стрессовых ситуациях, но также может препятствовать активации необходимых физиологических механизмов в ответ на проблемы.
2) Объяснены функциональные тесты на нейротрансмиттере
Функциональные тесты на нейротрансмиттере предназначены для изучения генетических факторов, которые влияют на то, как нейротрансмиттеры синтезируются, высвобождаются и разбиты в мозге. Поскольку нейротрансмиттеры являются химическими посланниками, которые регулируют настроение, познание и стрессовые реакции, различия в генах, связанных с этими веществами, могут иметь значительные последствия для психологической устойчивости человека и общего психического здоровья.
Изучение функции нейротрансмиттера в контексте устойчивости и реакции на стресс выходит за рамки только катехоламинов, таких как дофамин. Он включает в себя более широкий спектр нейротрансмиттерных систем, участвующих в регуляции настроения, бдительности и когнитивной функции. Несколько генов участвуют в синтезе, передаче сигналов и расщеплении этих нейротрансмиттеров, включая дофаминовую бета-гидроксилазу (DBH), дофаминовые рецепторы (DRD2, DRD4) и различные гены, связанные с серотонином.(13)
Комт (катехол-о-метилтрансфераза)
Ген COMT является одним из наиболее широко изученных в отношении функции нейротрансмиттера. Он кодирует фермент, который разрушает катехоламины, такие как дофамин, адреналин и норэпинефрин - нейротрансмиттеры, критические для реакции на стресс, познания и регуляции эмоций.
В гене COMT существуют хорошо известные полиморфизмы, такие как вариант Val158met. Этот вариант может повлиять на уровень активности фермента по -разному:(14-15)
- Форма высокой активности (вал валь): Эта форма фермента расщепляет дофамин более высокой скоростью, что может привести к более низким уровням дофамина в префронтальной коре, области мозга, участвующего в исполнительной функции и принятии решений. Люди с этим вариантом могут лучше работать в задачах в стабильных условиях, но могут быть более восприимчивы к снижению когнитивных средств при стрессе из -за более низкой доступности дофамина.
- Форма с низкой активностью (вариант MET): И наоборот, эта форма фермента COMT метаболизирует дофамин более медленно, что приводит к более высоким уровням дофамина в мозге. Это может усилить когнитивную функцию при стрессе, но также может увеличить риск психопатологии, связанных с стрессом, таких как тревожные расстройства, поскольку мозг потенциально чрезмерно стимулируется избыточным дофамином.
Дофаминовая бета-гидроксилаза (DBH)
DBH-это фермент, который превращает дофамин в норэпинефрин, нейротрансмиттер, участвующий в ответе борьбы или полета. Генетические вариации в гене DBH могут влиять на активность фермента, влияя на уровни дофамина и норэпинефрина в мозге и периферической нервной системе.(16-18)
- Низкая активность: Варианты, связанные с более низкой активностью DBH, могут привести к более высоким уровням дофамина и снижению уровней норэпинефрина, что может повлиять на когнитивные функции, такие как внимание и принятие решений, и влиять на то, как человек реагирует на стресс.
- Высокая активность: И наоборот, варианты, которые приводят к более высокой активности DBH, могут снизить уровень дофамина при одновременном увеличении норэпинефрина, потенциально влияя на стресс -реактивность и тревогу.
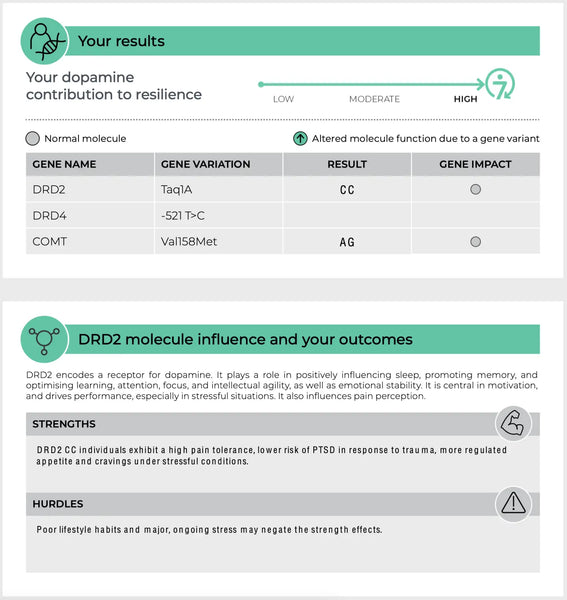
Дофаминовые рецепторы (DRD2 и DRD4)
DRD2 и DRD4 являются генами, которые кодируют дофаминовые рецепторы D2 и D4 соответственно. Эти рецепторы являются частью системы дофамина, которая регулирует многочисленные функции, включая настроение, вознаграждение и моторное управление.19-20)
- DRD2: Варианты в гене DRD2 могут влиять на плотность и аффинность связывания рецепторов D2. Это может влиять на уязвимость к стрессу и эффективности подсказок, связанных с вознаграждением, с потенциальными последствиями для таких состояний, как зависимость и депрессия.
- DRD4: Ген DRD4 известен переменным числом тандемных повторений (VNTR) в области кодирования. Конкретные повторения связаны с такими чертами, как поиск новинок и риск расстройств внимания. Этот полиморфизм может влиять на эффективность передачи сигналов синаптического дофамина и изучен в контексте поведенческой и психологической устойчивости.
Изображение: Пример страницы Тест ДНК устойчивости.
Вариации гена серотонина
Серотонин является еще одним важным нейротрансмиттером в регуляции настроения, аппетита и сна, на все из которых может влиять стресс. Несколько генов, участвующих в передаче сигналов серотонина, представляют интерес:
- 5-HTT (SLC6A4): Этот ген кодирует транспортер серотонина, ответственный за обратный захват серотонина из синаптической расщелины. Полиморфизм 5-HTTLPR в промоторной области этого гена был изучен на предмет его связи с чувствительностью к стрессу и расстройствами настроения.(21)
- Маоа: Моноаминоксидаза А является ответственным за разрушение серотонина. Изменения в этом гене могут влиять на уровни серотонина и связаны с поведенческими признаками и восприимчивостью к стрессу.(22)
- TPH2: Триптофан-гидроксилаза-2 является ферментом, критическим для синтеза серотонина в мозге. Генетические вариации в TPH2 могут влиять на выработку серотонина, потенциально влияя на эмоциональную регуляцию и реакцию на стресс.(23)
Интерпретация результатов этих тестов может быть сложной. Например, вариант, связанный с более высоким риском условий, связанных с стрессом, не обязательно означает, что у человека будут развиваться такие условия, но это может указывать на повышенную уязвимость. Точно так же обладание вариантом «устойчивости» не гарантирует непристойного сопротивления стрессу, поскольку факторы окружающей среды и выбор образа жизни играют существенные роли.
3) воспаление и его влияние на мозг
Хроническое воспаление может быть вредным для здоровья мозга. Считается, что он способствует развитию и прогрессированию нейродегенеративных заболеваний и может влиять на реакцию на стресс мозга, которая тесно связана с устойчивостью. Провоспалительные цитокины, такие как интерлейкин-6 (IL-6) и фактор некроза опухоли (TNF), могут преодолеть гематоэнцефалический барьер и взаимодействовать с путями, которые регулируют настроение, мотивацию и бдительность.(24)
Варианты генов, связанные с воспалением
IL-6 (интерлейкин-6)
IL-6-это цитокин, который играет роль в воспалительном ответе и участвует в различных биологических функциях, включая иммунный ответ, гематопоэз и метаболизм кости. В контексте стресса и устойчивости IL-6 связано со следующим:(25-26)
- Повышенные уровни IL-6 были связаны с большим риском развития депрессии и других расстройств настроения.
- Специфические генетические варианты гена IL-6 могут привести к повышению экспрессии IL-6, потенциально усугубляя воспалительный ответ и изменять способность мозга справляться со стрессом.
TNF (фактор некроза опухоли)
TNF является еще одним цитокином, участвующим в системном воспалении. Он играет различные роли, включая регуляцию иммунных клеток и индукцию лихорадки, апоптоза и воспаления.(27)
- Сверхэкспрессия TNF была связана с различными хроническими заболеваниями, включая ревматоидный артрит, воспалительное заболевание кишечника и псориаз, а также психологический стресс и депрессию.
- Варианты в гене TNF могут влиять на уровень продукции TNF, что может иметь прямые последствия для воспалительных процессов по всему организму и мозгу.
4) Нейробиологические регуляторы и факторы устойчивости
Генетические различия в важных нейробиологических регуляторах также регулируют устойчивость к стрессу. К ним относятся окситоцин, нейротрофический фактор, полученный из мозга (BDNF) и нейропептид Y (NPY). Окситоцин играет роль в регулировании социального поведения и стрессовых реакций. BDNF имеет решающее значение для поддержания нейропластичности, а когнитивная функция при стрессе, а NPY модулирует тревогу и физиологический стресс. Эти молекулы в совокупности влияют на нашу нервную схему и психологическую устойчивость, предоставляя представление об индивидуальных различиях в устойчивости стресса и потенциальных возможностях для персонализированных стратегий биохакирования.
Окситоцин
Окситоцин часто называют «любовным гормоном» из -за его роли в социальной связи, материнского поведения и близости. Он также оказывает анксиолитическое влияние и влияет на социальное познание и поведение.
- Генетическое влияние: Вариации в гене рецептора окситоцина (OXTR) были связаны с эмоциональной регуляцией, социальным поведением и отзывчивостью стресса. Специфические полиморфизмы в OXTR могут влиять на эффективность связывания и уровни экспрессии рецепторов окситоцина, что может повлиять на способность человека справляться с стрессом и формировать социальные отношения.(28)
- Подразумеваемое: В контексте тестирования устойчивости оценка вариантов OXTR может дать представление о предрасположенности человека к социальной тревоге, уровням эмпатии и потенциальной устойчивости в отношении стресса, особенно той, которая включает в себя социальную динамику.(29)
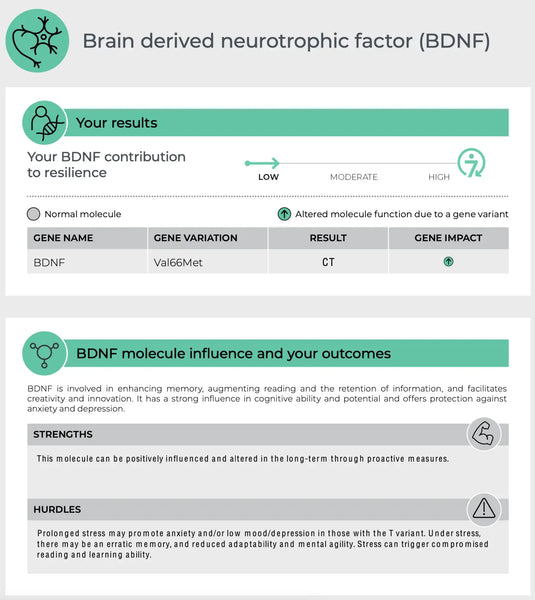
Нейротрофический фактор, полученный из мозга (BDNF)
BDNF является важным белком, участвующим в нейропластичности, способности мозга реорганизовать и формировать новые нейронные связи на протяжении всей жизни. Это важно для обучения, памяти и регенерации нейронов.(30)
- Генетические вариации: Вариант гена BDNF Val66met является одним из наиболее изученных полиморфизмов. Аллель MET был связан с пониженной активностью, зависящей от секреции BDNF, что может повлиять на когнитивную функцию и устойчивость к неврологическим и психическим расстройствам.(31)
- Подразумеваемое: Тестирование на варианты генов BDNF может помочь предсказать способность человека к нейропластичности в ответ на стресс и может иметь значение для восстановления после неврологических оскорблений или травм.(32)
Изображение: Пример страницы Анализ тестов на устойчивость ДНК.
Нейропептид Y (NPY)
NPY является одним из наиболее распространенных пептидов в мозге и участвует в регулировании стрессовых реакций, тревожности и потребления пищи. Это считается эндогенным анксиолитическим агентом.
- Генетические ассоциации: Полиморфизмы в гене NPY могут привести к различиям в экспрессии и секреции NPY, что потенциально влияет на устойчивость к стрессу человека. Более высокие уровни NPY, как правило, защищают от воздействия стресса.
- Подразумеваемое Генетическое тестирование для вариантов NPY может потенциально указывать на базовую устойчивость стресса человека и может предсказать их психологический ответ на хронический стресс и травму.(33)
Роль эпигенетики в модулировании устойчивости стресса
Поле Эпигенетика углубляется в слой сложности за пределами статической последовательности ДНК. Эпигенетические модификации состоят из химических изменений в структуре ДНК, таких как метилирование и модификации гистонов, которые могут влиять на то, как гены экспрессируются без изменения основного генетического кода. Эти изменения динамичны и реагируют на стимулы окружающей среды, включая хронический стресс. Воздействие длительного стресса может привести к эпигенетическим изменениям, которые влияют на функционирование генов, участвующих в реакции на стресс, что потенциально затрудняет эффективное обращение с новыми стрессорами.(34-35)
Компании, находящиеся в авангарде персонализированной медицины, в настоящее время становятся в эпигенетику, стремясь раскрыть, как эти модификации могут способствовать способности человека противостоять и отскочить от стресса. Анализируя эпигенетические маркеры, ученые могут получить представление о текущем состоянии профилей экспрессии генов, которые являются результатом как генетической предрасположенности, так и влияния окружающей среды. Такие тесты могут идентифицировать эпигенетические изменения в ответ на хронический стресс, обеспечивая снимок молекулярных механизмов, способствующих реакции на стресс человека или его отсутствия.
Кроме того, понимание этих эпигенетических изменений открывает дверь для персонализированных вмешательств. Конкретные изменения образа жизни, корректировки питания и терапевтические стратегии могут быть рекомендованы для обращения неблагоприятных эпигенетических модификаций. Этот персонализированный подход не только повышает устойчивость человека, но и способствует более широкому пониманию того, как стресс влияет на наши биологические системы на эпигенетическом уровне, что может привести к новым методам лечения и профилактическим стратегиям расстройств, связанных с стрессом. Картивая эпигенетический ландшафт реакции на стресс, мы можем начать рисовать более полную картину факторов, которые способствуют нашей уникальной способности справляться с жизненными проблемами.
Преимущества и ограничения тестов ДНК устойчивости
Эти тесты могут дать людям лучшее понимание их врожденной устойчивости и реакции на стресс. Такие знания могут проинформировать персонализированные стратегии для управления стрессом и улучшения психического здоровья. Тем не менее, важно подходить к этим тестам с тщательностью:
- Сложная черта: Устойчивость не определяется одним геном, но является сложной чертой, под влиянием многих генов и факторов окружающей среды.
- Влияние на окружающую среду: Генетика не работает в изоляции. Опыт жизни, системы поддержки и выбор образа жизни важны для устойчивости.
- Прогнозирующая сила: Хотя эти тесты могут дать информацию, они не являются окончательными предикторами способности человека справляться с жизненными проблемами.
Заключение
Тесты ДНК, связанные с устойчивостью, представляют собой интригующую границу в понимании того, как наш генетический состав может влиять на способность справляться с стрессом и восстанавливаться от невзгод. Хотя они могут дать ценную информацию, их следует рассматривать как одну часть гигантской головоломки, которая составляет устойчивость человека. Для тех, кто интересуется этими тестами, рекомендуется проконсультироваться с поставщиком здравоохранения или генетическим консультантом, чтобы интерпретировать результаты в более широком контексте их здоровья и образа жизни.
- Получите свой интегральный тест ДНК здесь!
- Забронируйте консультацию, чтобы интерпретировать ваши лаборатории и генные тесты с доктором Совиджарви здесь.
Научные ссылки:
- Bobrow, M. & Grimbaldeston, A.H. (2000). Медицинская генетика, Проект генома человека и общественное здравоохранение: представлено в Университете Ливерпуля, 9 декабря 1996 года и принято для публикации 17 февраля 2000 года. Журнал эпидемиологии и общественного здравоохранения, 54 (9), 645-649.
- Cicchetti, D. (2010). Устойчивость в условиях крайнего стресса: многоуровневая перспектива. World Psychiatry, 9 (3), 145.
- Luecken, L.J. & Gallo, L.C. (Eds.). (2008). Справочник по методам физиологического исследования в области психологии здоровья. Мудрец.
- Саутвик, С. М., Витилингем, М. и Чарни, Д. С. (2005). Психобиология депрессии и устойчивость к стрессу: последствия для профилактики и лечения. Анну. Преподобный клин. Psychol., 1, 255-291.
- Michaud, M., Balardy, L., Moulis, G., Gaudin, C., Peyrot, C., Vellas, B., ... & Nourhashemi, F. (2013). Провоспалительные цитокины, старение и возрастные заболевания. Журнал Американской ассоциации медицинских директоров, 14 (12), 877-882.
- Aguilera, G., Nikodemova, M., Wynn, P.C., & Catt, K.J. (2004). Кортикотропин рецепторы выпуска гормонов: два десятилетия спустя. Пептиды, 25 (3), 319-329.
- Subbannayya, T., Balakrishnan, L., Sudarshan, G., Advani, J., Kumar, S., Mahmood, R., ... & Prasad, T.K. (2013). Интегрированная карта передачи сигнального пути, релезинного кортикотропина. Журнал сотовой связи и передачи сигналов, 7, 295-300.
- Биндер Э. Б. (2009). Роль FKBP5, совместно-хаперона рецептора глюкокортикоидов в патогенезе и терапии аффективных и тревожных расстройств. Psychoneuroendocrinology, 34, S186-S195.
- Ising, M., Depping, A.M., Siebertz, A., Lucae, S., UNSCHULD, P.G., Kloiber, S., ... & Holsboer, F. (2008). Полиморфизмы в области гена FKBP5 модулируют восстановление после психосоциального стресса в здоровых контролях. Европейский журнал нейробиологии, 28 (2), 389-398.
- Vitellius, G., Trabado, S., Bouligand, J., Delemer, B. & Lombès, M. (2018, июнь). Патофизиология передачи сигналов глюкокортикоидов. В Annales d'Endocrinologie (том 79, № 3, с. 98-106). Elsevier Masson.
- Van West, D., Van Den Eede, F., Del-Favero, J., Souery, D., Norrback, K.F., Van Duijn, C., ... & Claes, S. (2006). Анализ SNP на основе гена на основе геновых рецепторов у пациентов с рецидивирующей большой депрессией. Нейропсихофармакология, 31 (3), 620-627.
- Schneider, K.K., Frings, C., Meyer, J. & Schote, A.B. (2016). Роль гена рецептора глюкокортикоидов (NR3C1) для обработки неприятных стимулов. Нейробиологические исследования, 107, 8-13.
- Azadmarzabadi, E., Haghighatfard, A. & Mohammadi, A. (2018). Низкая устойчивость к стрессу связана с изменениями экспрессии генов кандидата в дофаминергическом сигнальном пути. Психогериатрия, 18 (3), 190-201.
- ЧАСEinz, A. & Smolka, M.N. (2006). Влияние генотипа катехольной о-метилтрансферазы на активацию мозга вызвано аффективными стимулами и когнитивными задачами. Обзоры в Neurosciences, 17 (3), 359-368.
- Mier, D., Kirsch, P., & Meyer-Lindenberg, A. (2010). Нейронные субстраты плейотропного действия генетического вариации в КоМТ: метаанализ. Молекулярная психиатрия, 15 (9), 918-927.
- Mustapic, M., Maihofer, A. X., Mahata, M., Chen, Y., Baker, D.G., O'Connor, D.T. & Nievergelt, C.M. (2014). Катехоламиновый биосинтетический фермент дофамин β-гидроксилаза (DBH): первые варианты поиска по всему геному. Молекулярная генетика человека, 23 (23), 6375-6384.
- Винсент С. и Робертсон Д. (2002). Более широкий взгляд: аномалии катехоламина. Клиническое вегетативное исследование, 12, I44-I49.
- Hoenicka, J., Aragüés, M., Ponce, G., Rodríguez-Jiménez, R., Jiménez-Arriero, M.A. & Palomo, T. (2007). От дофаминергических генов до психиатрических расстройств. Исследование нейротоксичности, 11, 61-71.
- Hill, S. Y., Hoffman, E.K., Zezza, N., Thalamuthu, A., Weeks, D.E., Matthews, A.G. & Mukhopadhyay, I. (2008). Дофаминергические мутации: ассоциация внутри семьи и связь в семействах мультиплексной алкогольной зависимости. Американский журнал медицинской генетики, часть B: нейропсихиатрическая генетика, 147 (4), 517-526.
- He, Y., Martin, N., Zhu, G. & Liu, Y. (2018). Гены кандидатов для поиска новизны: мета-анализ ассоциаций исследований: DRD4: Exon III и: COMT: Val158met. Психиатрическая генетика, 28 (6), 97-109.
- Avula, R., Rand, A., Black, J.L. & O'Kane, D.J. (2011). Одновременное генотипирование множественных полиморфизмов в гене транспортера серотонина человека и обнаружение новых аллельных вариантов. Трансляционная психиатрия, 1 (8), E32-E32.
- Tivol, E.A., Shalish, C., Schuback, D.E., Hsu, Y.P. & Breakefield, X. O. (1996). Мутационный анализ гена Маоа человека. Американский журнал медицинской генетики, 67 (1), 92-97.
- Waider, J., Araragi, N., Gutknecht, L. & Lesch, K.P. (2011). Триптофан-гидроксилаза-2 (TPH2) при расстройствах когнитивного контроля и регуляции эмоций: перспектива. Psychoneuroendocrinology, 36 (3), 393-405.
- Bauer, M.E. & Teixeira, A.L. (2021). Нейроинфляция при расстройствах настроения: роль регуляторных иммунных клеток. Нейроиммуномодуляция, 28 (3), 99-107.
- Anderson, G., Kubera, M., Duda, W., Lasoń, W., Berk, M. & Maes, M. (2013). Увеличение транс-сигнала IL-6 в депрессии: сосредоточиться на пути катаболита триптофана, мелатонина и нейропрогрессии. Фармакологические отчеты, 65 (6), 1647-1654.
- Perry, B.I., Upthegrove, R., Kappelmann, N., Jones, P.B., Burgess, S. & Khandaker, G.M. (2021). Ассоциации иммунологических белков/признаков с шизофренией, большой депрессией и биполярным расстройством: двунаправленное исследование рандомизации с двумя выборками. Мозг, поведение и иммунитет, 97, 176-185.
- Брэдли Дж. (2008). TNF -опосредованное воспалительное заболевание. Журнал патологии: журнал Патологического общества Великобритании и Ирландии, 214 (2), 149-160.
- ЛOth, E., Poline, J. B., Thyreau, B., Jia, T., Tao, C., Lourdusamy, A., ... & Imagen Consortium. (2014). Генотип рецептора окситоцина модулирует вентральную стриатальную активность в социальных сигналах и реакции на стрессовые жизненные события. Биологическая психиатрия, 76 (5), 367-376.
- Myers, A.J., Williams, L., Gatt, J.M., Mcauley-Clark, E. Z., Dobson-Stone, C., Schofield, P.R. & Nemeroff, C.B. (2014). Различия в гене рецептора окситоцина связаны с повышенным риском тревоги, стресса и депрессии у людей с историей воздействия на ранней жизни стресса. Журнал психиатрических исследований, 59, 93-100.
- Cowansage, K.K., Ledoux, J.E. & Monfils, M.H. (2010). Нейротрофический фактор, полученный из мозга: динамический привратник нейронной пластичности. Текущая молекулярная фармакология, 3 (1), 12-29.
- Chen, Z. Y., Jing, D., Bath, K.G., Ieraci, A., Khan, T., Siao, C.J., ... & Lee, F. S. (2006). Генетический вариант BDNF (VAL66MET) полиморфизм изменяет поведение, связанное с тревогой. Science, 314 (5796), 140-143.
- Филлипс, С. (2017). Нейротрофический фактор, депрессия и физическая активность, полученный из мозга: установление нейропластической связи. Нейронная пластичность, 2017.
- Schmeltzer, S.N., Herman, J.P. & Sah, R. (2016). Нейропептид Y (NPY) и посттравматическое стрессовое расстройство (ПТСР): переводное обновление. Экспериментальная неврология, 284, 196-210.
- Lux, V. (2016, март). Эпигенетическое программирование в психобиологическом развитии: доказательства теории двойной активации и посреднической роли стресса ранней жизни. В ходе ежегодных исследовательских конференций в Катарском фонде Том 1 выпуск 1 (том 2016, № 1, стр. HBOP3415). Hamad Bin Khalifa University Press (Hbku Press).
- Chukwuma Sr, C. (2022). Эпигенетика и ее сущность в понимании роста, развития и болезней человека. J Med Res, 8 (5), 165-172.