Everything you need to know aboout aging and anti-aging processes. How to reverse aging? What are the 9 hallmarks of aging? Read more to find out!
In 2013, Carlos López-Otín and his colleagues published a ground-breaking paper, where they attempted to identify and categorize the cellular and molecular hallmarks of aging. They proposed nine candidate hallmarks that are generally considered to contribute to the aging process and together determine the aging phenotype. López-Otín et al. defined that a hallmark should ideally fulfill the three following criteria: it should manifest during normal aging, its experimental aggravation should accelerate aging and its experimental amelioration should retard the normal aging process and hence increase healthy lifespan.
The original nine hallmarks of aging
The key to slowing down or even reversing aging is not based on the idea of just increasing lifespan, but healthspan (the period of life spent in good health, free from the chronic diseases and disabilities of aging). There hasn’t been that much compression of morbidity to date because we have reduced mortality more than we prevented morbidity. Healthspan (having healthy years of life) will be increased when morbidity (the condition of suffering from a disease or medical condition) is decreased, most effectively through raising the age of onset.
Successful and healthy aging can be defined as follows:
- Low probability of disease or disability
- High cognitive and physical function capacity
- Active engagement with life
The proposed nine hallmarks of aging are grouped into three categories:
- 1. Primary hallmarks (causes of damage)
- The initiating triggers whose damaging consequences progressively accumulate with time. All unequivocally negative.
- 2. Antagonistic hallmarks (responses to damage)
- Opposite effects to the primary hallmarks. At low levels, they mediate beneficial effects, but at high levels, they become deleterious (e.g. senescence).
- 3. Integrative hallmarks (culprits of the phenotype)
- The end result of the previous two groups of hallmarks and accumulated damage that cannot be compensated by tissue homeostatic mechanisms. These are ultimately responsible for the functional decline associated with aging.
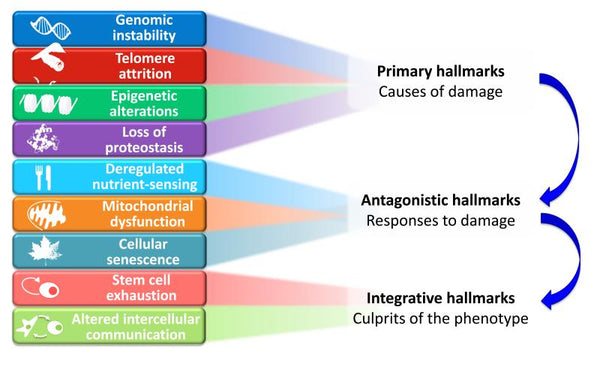
Image: Functional Interconnections between the Hallmarks of Aging.
Source: López-Otín, C. & Blasco, M. & Partridge, L. & Serrano, M. & Kroemer, G. (2013). The hallmarks of aging. Cell 153 (6): 1194–1217.
1. Genomic instability
The integrity and stability of DNA are continuously challenged by external physical, chemical, and biological agents, as well as by internal threats, that include DNA replication errors, spontaneous hydrolytic reactions, and the production of reactive oxygen species. These eventually lead to the accumulation of genetic damage throughout life.
Ways to improve genomic stability (Hallmark #1):
- Antioxidative micronutrients (selenium, zinc, vitamin C, vitamin E) -> limit DNA lesions
- Fix even slight deficiencies in folate, vitamin B12, niacin and zinc -> impact significantly on spontaneous chromosome damage rate
- Ganoderma lucidum (reishi) glucans
- Polyphenols -> scavenge free radicals
- Moderate & ”auto-regulated” exercise
- Avoid the following:
- Radiation & chemical exposure
- Heavy metal exposure
- Nickel, cadmium & arsenic, in particular
- Cigarette smoking
- Sitting too long
- Excessive use of alcohol, energy drinks & milk
2. Telomere attrition
Telomeres are particularly susceptible to age-related deterioration. Telomere shortening is observed during normal aging both in humans and in mice. Telomeres are bound by a multiprotein complex called shelterin, which prevents the access of DNA repair proteins to the telomeres (without it telomeres would be “repaired” as DNA breaks leading to chromosome fusions). DNA damage at telomeres is remarkably constant and highly efficient in inducing senescence.
Ways to reduce telomere shortening (Hallmark #2):
- Increase dietary antioxidant intake
- High omega-3 fatty acid intake, in particular
- Optimize vitamin D, vitamin B12, and folate levels in the blood
- Mediterranean diet
- Avoid the following:
- Smoking
- Obesity
- Toxin exposure & pollution
- Balance stress levels
- Balance exercise routine and intense bouts of exercise
- Loving-kindness meditation & mindfulness meditation practice (and meditation in general)
- Hyperbaric oxygen therapy (HBOT)
- Gynostemma, gotu kola & astragalus herbs (possibly effective)
- What does not work: long-term caloric restriction
3. Epigenetic alterations
Many kinds of epigenetic alterations affect all cells and tissues throughout life (caused by things such as diet, chemicals, drugs, sunlight, heat/cold, exercise, etc.). Epigenetic changes presuppose alterations in DNA methylation patterns, posttranslational modification of histones, and chromatin remodeling. Members of the sirtuin family of NAD-dependent protein deacetylases and ADP ribosyltransferases have been studied extensively as potential anti-aging factors – in humans, at least three members of the sirtuin family, SIRT1, SIRT3, and SIRT6, contribute to healthy aging.
Ways to manage epigenetic alteration (Hallmark #3):
- Optimize methylation pathways (folate, B12, B6, TMG)
- Sirtuin pathways activators (SIRT1, SIRT3, SIRT6):
- Spermidine (inhibits histone deacetylases)
- Intermittent calorie restriction & intermittent fasting
- Regular (intense) exercise
- Sleep optimization (sleep loss causes harmful epigenetic alterations)
- Metformin (side effects are the caveat); preferably (dihydro)berberine
- Alpha-ketoglutarate (CaAKG)
- Get regularly hormetic doses of heat, cold, sunlight, etc. (”poison is in the dose”)
- Epigenetic drugs in development that target DNA methyltransferase, histone deacetylase, histone methyltransferase, and chromatin
Recommended supplements:
Purovitalis Liposomal Quercetin
Purovitalis Liposomal Resveratrol
4. Loss of proteostasis
Proteostasis comprises mechanisms for the stabilization of correctly folded proteins (especially the heat-shock family of proteins) and mechanisms for the degradation of proteins by the proteasome or the lysosome. Multiple studies have demonstrated that proteostasis is altered with aging leading to chronic expression of unfolded, misfolded, or aggregated proteins. These contribute to the development of some age-related degenerative diseases, such as Alzheimer’s disease.
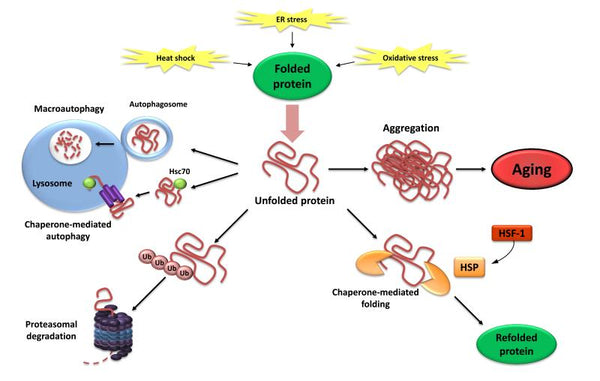
Image: Loss of Proteostasis. Failure to refold or degrade unfolded proteins can lead to their accumulation and aggregation, resulting in proteotoxic effects.
Source: López-Otín, C. & Blasco, M. & Partridge, L. & Serrano, M. & Kroemer, G. (2013). The hallmarks of aging. Cell 153 (6): 1194–1217.
Ways to improve proteostasis stability (Hallmark #4):
In general, the best way to protect the body from the loss of proteostasis is by activating autophagy.
- Fasting, intermittent calorie restriction (long-term is not useful!), ketosis, exercise (on a fasted state the best), resistance training, cold & heat exposure, deep sleep (and melatonin)
- Sulforaphane, coffee, curcumin, polyphenols, EVOO, resveratrol, green tea, spermidine
- Senolytic nutrients & supplements:
- Quercetin, fisetin, luteolin, curcumin, piperlongumine, molecular hydrogen (H2) etc.
- AMPK activators:
- Berberine (DHB even better), resveratrol (and pterostilbene), hesperidin, ginsenosides, quercetin, curcumin, naringenin, gynostemma, crocin (in saffron), salvianolic acid B, alpha-lipoic acid, etc.
- Metformin & rapamycin (Nb. possible side effects)
- Hsp70 (heat-shock protein 70) inducers:
- Sauna and heat exposure
- Shikonin (from the roots of the shikonin plant)
- Specific senolytic drugs (such as Dasatinib and Navitoclax)
5. Deregulated nutrient sensing
IGF-1 and insulin signaling is known as the IIS pathway, which is the most conserved aging-controlling pathway in evolution. In addition to the IIS pathway that takes part in glucose sensing, there are three additional related and interconnected nutrient-sensing systems: mTOR (sensing of high amino acid concentrations), AMPK (sensing of low-energy states by detecting high AMP levels), and sirtuins (sensing low-energy states by detecting high NAD+ levels). To summarize, there is strong evidence that anabolic signaling (mTOR, high insulin) accelerates aging and decreased nutrient signaling (AMPK, low insulin) extends longevity.
Ways to manage deregulated nutrient sensing (Hallmark #5):
Practically, all of the previous ways to manage hallmarks 1-4 are covered in sensitizing and re-regulating nutrient sensing. These include:
- dietary restrictions
- AMPK activation
- sirtuin activation
- hormesis
- reducing oxidative stress and inflammation
- increasing autophagy
6. Mitochondrial dysfunction
Mitochondrial dysfunction has been found to increase the aging process. When an organism ages the efficacy of the cells’ respiration chain diminishes, which leads to electron leakage and reduced ATP generation. The reduced efficiency of mitochondrial bioenergetics with aging may result from multiple intersecting mechanisms, including reduced biogenesis of mitochondria, accumulation of mutations and deletions in mtDNA, oxidative stress in mitochondrial proteins, destabilization of the respiratory chain, changes in the lipid composition of mitochondrial membranes and alterations in mitochondrial dynamics.
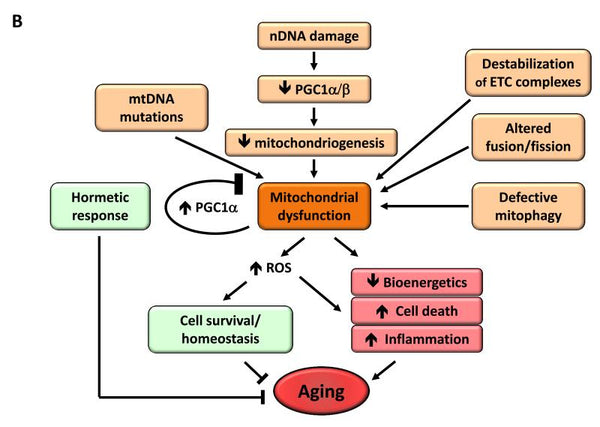
Image: Mitochondrial dysfunction and its effect on aging.
Source: López-Otín, C. & Blasco, M. & Partridge, L. & Serrano, M. & Kroemer, G. (2013). The hallmarks of aging. Cell 153 (6): 1194–1217.
Ways to manage mitochondrial dysfunction (Hallmark #6):
- Activate mitohormesis with:
- Intermittent calorie restriction
- Intermittent fasting
- Exercise
- Cold exposure
- Phytonutrients (such as flavonoids)
- Target the Nrf2 pathway (homeostasis & structural integrity) with:
- Heat & cold exposure
- Physical exercise
- Intermittent fasting and ketosis
- Sulforaphane, polyphenols, quercetin, curcumin, etc.
- Molecular hydrogen (H2) -> product recommendation
- Improve mitochondrial biogenesis & repair (activating PGC-1α):
- Physical exercise
- Ketosis and ketone bodies
- Acetyl-L-carnitine
- Polyphenols, quercetin
- Rhodiola rosea (salidrosides)
- Coenzyme Q10, pyrroloquinoline quinone (PQQ), nicotinamide mononucleotide (NMN), alpha-lipoic acid (ALA)
7. Cellular senescence
Because the number of senescent cells increases with aging, it has been presupposed that senescence contributes to aging. Senescence is however needed to prevent the distribution and proliferation of damaged cells triggering an immune system response. This cellular checkpoint requires an efficient cell substitution system that involves both clearances of senescent cells and mobilization of progenitor cells to restore optimal cell numbers. Senescent cells express substantial alterations in their secretome, which is particularly enriched in proinflammatory cytokines and matrix metalloproteinases. It is hence referred to as the “senescence-associated secretory phenotype”.
Ways to combat cellular senescence (Hallmark #7):
Senescent cells eventually stop multiplying but don’t die off when they should, as regular cells do. Instead, they remain and continue to release chemicals that can trigger inflammation and immune response.
Senolytic compounds that selectively target senescent cells include:
- Fisetin (also called “the ultimate senolytic”)
- Quercetin
- Theaflavins
- Apigenin
- Tocotrienols (form of vitamin E)
- Piperlongumine
- Molecular hydrogen (H2)
8. Stem cell exhaustion
Adult stem cells are able to self-renew and differentiate into multiple cell types within a tissue. Although phenotypes and mechanisms vary widely, all stem cell populations decline in function with age. Stem cell depletion is the unifying consequence of various aging-associated damages and likely constitutes one of the ultimate culprits of cellular aging. Studies on aged mice have revealed an overall decrease in the cell-cycle activity of hematopoietic stem cells (HSCs), which correlates with the accumulation of DNA damage and overexpression of cell-cycle inhibitory proteins (e.g. p16INK4a). Telomere shortening has also been found as an important cause of stem cell decline in aging.
Ways to counteract stem cell exhaustion (Hallmark #8):
- Stem cell therapy (autologous vs allogeneic & various cell types)
- Stem cell therapies are still a ”wild west” – potential side effects and risks are there, such as the growth of tumors and cancer.
- Photobiomodulation (”red light therapy”)
- PBM stimulates different types of stem cells to enhance their migration, proliferation, and differentiation in vitro and in vivo.
- Restore aged stem cells by targeting toxic metabolites:
- Activate sirtuins – SIRT1 & SIRT 3 (see #3 & #4)
- N-acetyl cysteine (NAC)
- Improve proteostasis stability (see #4
- Improve mitochondrial function (see #6)
- Use senolytics (see #7)
- Restore epigenetic memory (see #3)
- Optimize vitamin D levels
- Spirulina (in vitro study)
9. Altered intercellular communication
Cellular aging takes place also at the level of intercellular communication. These include neurohormonal signaling (increased inflammatory reactions), immunosurveillance (pathogens and premalignant cells) and extracellular environment changes. Aging due to inflammation is called inflammaging. It may result from multiple causes, such as the accumulation of proinflammatory tissue damage, the failure of a dysfunctional immune system to effectively clear pathogens and dysfunctional host cells and the occurrence of a deficient autophagic response. Aging changes in one tissue can lead to aging-specific deterioration of the neighbouring tissue: senescent cells induce senescence in their adjacent cells via gap-junction-mediated cell-cell contacts and processes involving reactive oxygen species. This phenomenon is also called a sensecent cell bystander effect.
Ways to improve intercellular communication (Hallmark #9):
- Lower general inflammation in the body
- An overall anti-inflammatory lifestyle approach
- Use molecular hydrogen (H2)
- Multiple anti-aging effects in the body (including the reduction of inflammaging)
- Lower oxidative stress in the body and reduce reactive oxygen species (ROS)
- Increase autophagy in the body (see before)
- Treat gut dysbiosis and improve the diversity and general health of the gut microbiota
- Treat possible leaky gut syndrome
10. Extracellular matrix (ECM) stiffness
In addition to these 2013 defined nine hallmarks of aging, there is a tenth hallmark in the making. Two researchers, Alexander Fedintsev and Alexey Moskalev, have published a paper in 2021 that takes a closer look at extracellular matrix (ECM) stiffening: the build-up of cross-links between long-lived molecules such as collagen and elastin. They suggest that ECM stiffening is caused by the non-enzymatic chemical reactions of glycation, carbamylation and carbonylation and that it could even be the upstream cause of several accepted hallmarks of aging, such as cellular senescence. These changes lead to the formation of adducts and cross-links that in turn cause inflammation, fibrosis, tissue circadian clock impairment, stem cell aging and so on. It has been previously established that advanced glycation end products (AGEs) have pathogenic significance for various tissues and pathways in the body. Organisms with extraordinarily long lifespans (such as bowhead whales) have exceptionally low rates of AGEs accumulation.
Potential therapeutics for extracellular matrix (ECM) stiffness (Hallmark #10):
In human skeletal muscles, the age-related functional impairment is due to increased stiffness of ECM, mainly caused by collagen accumulation.
- Manual therapy and mechanistic pressure
- Osteopathic therapy, acupuncture, myofascial release
- A combination of synthetic and natural AGE inhibitors working synergistically at different stages of formation
- Natural products include carnosine, alpha-lipoic acid, taurine, vitamin C, benfotiamine and pyridoxamine -> product recommendation
- Polyphenols, terpenoids and polysaccharides
- Also, reduce the intake of advanced glycation end-products from food
- Stimulate elastogenesis (new drugs?)
- RAGE (receptor for advanced glycation end-products) antagonists [different peptides]
///








